Polycythemia Vera Market is expected to Reach USD 5,257 million by 2034
Get a Sneak Peek at the Latest polycythemia vera market size Report
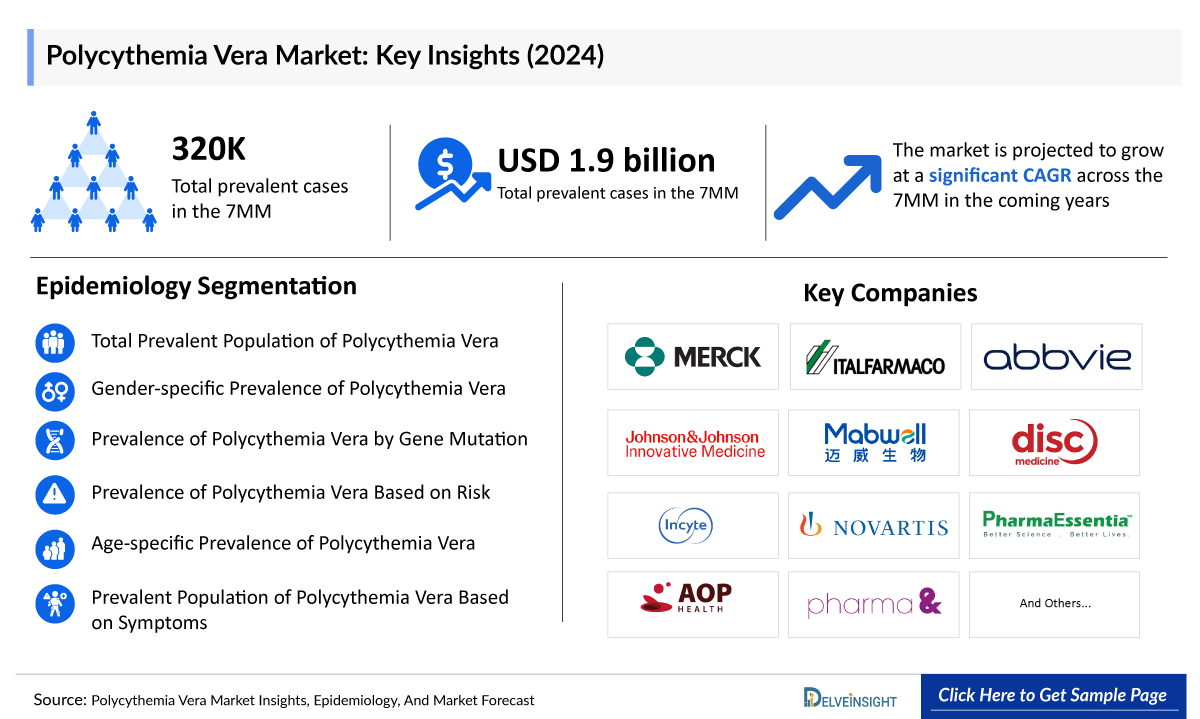
Polycythemia Vera market in the 7 major markets (7MM) is projected to grow significantly from USD 2,087 million in 2025 to USD 5,257 million by 2034, reflecting a CAGR of 10.8%.
In 2024, the Polycythemia Vera market size was approximately USD 1,900 million, with the United States holding the largest share. Among existing treatments, JAKAFI (ruxolitinib) generated the highest revenue (~USD 965 million in 2024). PV remains an incurable condition, but current treatments like JAKAFI and BESREMi (ropeginterferon alfa-2B) help manage symptoms and complications. Both are approved across the US, Europe, and Japan.
BESREMi’s broad label gives PharmaEssentia flexibility in positioning it across multiple therapy lines, potentially reducing the need for JAKAFI. Meanwhile, rusfertide, with a novel mechanism of action and promising results in reducing phlebotomy frequency, could become a new standard of care post-2027 following expected approval in Q4 2026. Additionally, several emerging therapies - including Bomedemstat (Merck), Givinostat (Italfarmaco), Divesiran (Silence Therapeutics), Sapablursen (Ionis Pharmaceuticals), and PPMX-T003 (Perseus Proteomics) - are expected to further expand the PV treatment landscape and drive market growth.
DelveInsight’s “Polycythemia Vera Market Insights, Epidemiology, and Market Forecast-2034″ report offers an in-depth understanding of the Polycythemia, historical and forecasted epidemiology as well as the Polycythemia market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Polycythemia market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Polycythemia Market Forecast
Some of the key facts of the Polycythemia Market Report
- The Polycythemia market size was valued approximately USD 1900 million in the 7MM in the year 2024 and is anticipated to grow with a significant CAGR during the study period (2020-2034)
- In August 2025, Protagonist Therapeutics’ rusfertide has been granted FDA Breakthrough Therapy Designation for the treatment of erythrocytosis in polycythemia vera, backed by encouraging Phase 3 VERIFY trial results presented at ASCO 2025.
- In August 2025, Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today announced the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation for VGT-1849B, a selective peptide nucleic acid-based JAK2 inhibitor for the treatment of polycythemia vera (PV).
- In May 2025, Italfarmaco S.p.A. announced today that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to givinostat for the treatment of patients with polycythemia vera (PV), a rare haematologic cancer, for which treatment options are limited.
- In May 2025, The FDA has granted Fast Track designation to givinostat (Duvyzat) for the treatment of Polycythemia Vera, according to a press release from its developer, Italfarmaco. Previously, givinostat received orphan drug designation for the same indication from both the FDA and the European Medicines Agency (EMA). Additionally, the drug has been approved by the FDA and the UK’s Medicines and Healthcare Products Regulatory Agency for the treatment of Duchenne muscular dystrophy.
- In March 2025, Protagonist Therapeutics, Inc. (NASDAQ: PTGX) and Takeda (TSE: 4502/NYSE: TAK) have announced positive topline results from the Phase 3 VERIFY study. The trial involved phlebotomy-dependent Polycythemia Vera (PV) patients, who were randomly assigned to receive either rusfertide or a placebo alongside standard care. The study successfully achieved its primary endpoint along with all four key secondary endpoints. Rusfertide, an investigational first-in-class hepcidin mimetic peptide therapeutic, has been granted Orphan Drug and Fast Track designations by the U.S. Food and Drug Administration (FDA).
- In December 2024, Silence Therapeutics plc ("Silence" or the "Company") (Nasdaq: SLN), a global clinical-stage company focused on developing innovative siRNA (short interfering RNA) therapies, today announced the presentation of additional results from the Phase 1 open-label segment of the SANRECO study of divesiran, a siRNA targeting TMPRSS6, in patients with Polycythemia Vera (PV) at the American Society of Hematology (ASH) Annual Meeting.
- In June 2024, AOP Orphan Pharmaceuticals GmbH (AOP Health) continues its successful hematology/oncology clinical research program with two abstracts accepted for presentation at the European Hematology Association (EHA) 2024 hybrid congress in Madrid, Spain. One abstract includes an oral presentation of the latest findings from the PROUD-PV and CONTINUATION-PV trials. The results demonstrate a link between genetic changes (molecular response) and event-free survival (EFS) in Polycythemia Vera (PV) patients treated with ropeginterferon alfa-2b (BESREMi®) or the best available treatment.
- In February 2024, Disc Medicine has disclosed that the US FDA has awarded Orphan Drug Designation to DISC-3405 for treating patients with PV. Additionally, in September 2023, the FDA granted fast track designation to MWTX-003, also known as DISC-3405, for PV treatment.
- In January 2024, Takeda and Protagonist Therapeutics have established a global licensing and collaboration agreement for rusfertide.
- In 2023, the United States had the highest number of cases among the 7MM. The total number of prevalent PV cases in the US in 2023 was approximately 180,000.
- In the EU4 and the UK, Germany had the highest number of PV cases, with nearly 25,000 cases in 2023, whereas Spain reported the lowest number of cases in the same year.
- As per Orphanet, PV affects 30 out of every 100,000 individuals in Germany. When extrapolated to the entire population, this translates to approximately 24,000 affected people.
- According to Kuykendall (2023), as many as 25% of patients develop resistance to or cannot tolerate hydroxyurea.
- Key Polycythemia Companies: Protagonist Therapeutics, Italfarmaco, Imago BioSciences, Silence Therapeutics, Ionis Pharmaceutical, Perseus Proteomics, Protagonist Therapeutics, Inc., Novartis, PharmaEssentia, AOP Orphan Pharmaceuticals AG, Incyte Corporation, and others
- Key Polycythemia Therapies: Rusfertide (PTG-300), Givinostat (ITF2357), Bomedemstat, SLN124, Sapablursen, PPMX-T003, PTG-300, SLN124, Hydroxyurea, Ropeginterferon alfa-2b, PEG-P-INF alpha-2b (P1101), Ruxolitinib, and others
- The Polycythemia epidemiology based on gender analyzed that mostly males are affected in case of Polycythemia
- · The Polycythemia market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Polycythemia pipeline products will significantly revolutionize the Polycythemia market dynamics.
Polycythemia Vera Overview
Polycythemia Vera is a rare blood disorder characterized by an increased number of red blood cells, leading to thicker blood and reduced blood flow. Polycythemia Vera (PV) is the most common type, classified as a myeloproliferative neoplasm and often associated with the polycythemia JAK2 mutation, which plays a crucial role in disease development. Polycythemia Vera symptoms typically include headache, dizziness, fatigue, itching (especially after a hot shower), and a ruddy complexion. In more severe cases, polycythemia complications can include blood clots, strokes, and heart attacks due to increased blood viscosity.
Polycythemia diagnosis involves blood tests to check hematocrit and hemoglobin levels, along with genetic testing for the polycythemia JAK2 V617F mutation. Polycythemia treatment aims to reduce red blood cell count and prevent complications through phlebotomy, low-dose aspirin, and in some cases, cytoreductive therapy such as hydroxyurea. Emerging therapies are focusing on targeting the molecular pathways of Polycythemia disease progression.
Raising awareness of polycythemia vera early detection and ensuring proper polycythemia vera management are essential for improving patient outcomes. As research continues to evolve, new polycythemia vera clinical trials and treatments offer hope for those living with this chronic condition. Regular monitoring and individualized care are key to effective polycythemia vera prognosis.
Polycythemia Vera Epidemiology
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2019 to 2032. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Polycythemia vera Epidemiology Segmentation
The Polycythemia market report proffers epidemiological analysis for the study period 2019–2032 in the 7MM segmented into:
- Total Prevalence of Polycythemia
- Prevalent Cases of Polycythemia by severity
- Gender-specific Prevalence of Polycythemia
- Diagnosed Cases of Episodic and Chronic Polycythemia
Download the report to understand which factors are driving Polycythemia epidemiology trends @ Polycythemia Epidemiology Forecast
Polycythemia Vera Therapies and Key Companies
- Rusfertide (PTG-300): Protagonist Therapeutics
- Givinostat (ITF2357): Italfarmaco
- Bomedemstat: Imago BioSciences
- SLN124: Silence Therapeutics
- Sapablursen: Ionis Pharmaceutical
- PPMX-T003: Perseus Proteomics
- PTG-300: Protagonist Therapeutics, Inc.
- SLN124: Silence Therapeutics plc
- Hydroxyurea: Novartis
- Ropeginterferon alfa-2b: PharmaEssentia
- PEG-P-INF alpha-2b (P1101): AOP Orphan Pharmaceuticals AG
- · Ruxolitinib: Incyte Corporation
Discover more about therapies set to grab major Polycythemia market share @ Polycythemia Vera Treatment Market
Polycythemia Vera Market Dynamics: Strengths and Opportunities
Polycythemia Vera Market Strengths
- The approval of BESREMi in the US and its expected approval in Japan, in both the first and second line of treatment, provides the interferon with an immense opportunity to garner a big market size in Polycythemia.
- Based on increasing sales of JAKAFI/JAKAVI, the drug will be a blockbuster therapy in 2L PV patients.
Polycythemia Vera Market Opportunities
- An increase in strategic alliances, such as geographical alliances, and granting the designation such as BTD and FTD by the FDA are some of the factors that will drive the market growth.
- Opportunity for drugs with novel and disease-modifying mechanisms and low side effect.
Scope of the Polycythemia Vera Market Report
- Study Period: 2020-2034
- Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
- Key Polycythemia Companies: Protagonist Therapeutics, Inc. (NASDAQ: PTGX), Italfarmaco, Imago BioSciences (formerly NASDAQ: IMGO, acquired by Merck), Silence Therapeutics (NASDAQ: SLN), Ionis Pharmaceuticals (NASDAQ: IONS), Perseus Proteomics (Tokyo Stock Exchange: 4882), Novartis (SWX: NOVN), PharmaEssentia (Taipei Exchange: 6446), AOP Orphan Pharmaceuticals AG, Incyte Corporation (NASDAQ: INCY), and others
- Key Polycythemia Therapies: Rusfertide (PTG-300), Givinostat (ITF2357), Bomedemstat, SLN124, Sapablursen, PPMX-T003, PTG-300, SLN124, Hydroxyurea, Ropeginterferon alfa-2b, PEG-P-INF alpha-2b (P1101), Ruxolitinib, and others
- Polycythemia Therapeutic Assessment: Polycythemia current marketed and Polycythemia emerging therapies
- Polycythemia Market Dynamics: Polycythemia market drivers and Polycythemia market barriers
- Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies
- Polycythemia Unmet Needs, KOL’s views, Analyst’s views, Polycythemia Market Access and Reimbursement
To know more about Polycythemia companies working in the treatment market, visit @ Polycythemia Clinical Trials and Therapeutic Assessment
Need more?
- ✅ Connect with our analyst to learn how this research was developed
- ✅ Expand the scope with additional segments or countries through free customization
- ✅ Discover how this report can directly influence your business growth
Related Reports
Polycythemia Vera Market Insight, Epidemiology and Market Forecast -2034
DelveInsight’s Polycythemia Vera Market Insights, Epidemiology and Market Forecast - 2034 report provides the detailed overview of the disease and in depth understanding of historical..
Polycythemia Vera - Pipeline Insight, 2025
Polycythemia Vera Pipeline Insights, 2025 report by DelveInsight outlays detailed insights of present clinical development scenario and growth prospects..
-Market-Vera-(PV)-Market-.png&w=256&q=75)

