Cardiac Amyloidosis Market
- The Cardiac Amyloidosis market is anticipated to sustain a steady Compound Annual Growth Rate (CAGR) during the forecast period (2024 to 2034). This growth in market revenue is chiefly propelled by advancements in diagnostic techniques, heightened awareness of the condition, and a growing number of reported cases.
- Cardiac amyloidosis is a substantially underdiagnosed disease. Improved awareness and advances in imaging over the past 2 decades have improved the noninvasive diagnosis of cardiac amyloidosis.
- Cardiac amyloidosis cases are increasing due to various factors including genetic mutation and other diseases such as multiple myeloma, among others.
- The future market presents numerous opportunities given the scarcity of available treatment options for the condition.
- The existing Cardiac amyloidosis market is characterized by less competition with limited market players including Pfizer, and others.
- Several companies including Alnylam Pharmaceuticals, Alexion Pharmaceuticals, Eidos Therapeutics, Ionis Pharmaceuticals, AstraZeneca, and others are developing their assets in the mid-late stage of development for the treatment of cardiac amyloidosis. With the expected approval of these therapies during the forecast period [2024–2034], the overall therapeutic market of cardiac amyloidosis is likely to witness a rise at a significant CAGR.
Factors affecting Cardiac Amyloidosis Market Growth
-
Rising Prevalence of Amyloidosis and Cardiovascular Diseases
Increasing cases of systemic amyloidosis and related cardiovascular complications are a primary driver for the cardiac amyloidosis market. Growing awareness of the disease’s impact on heart function is fueling demand for early diagnosis and treatment.
-
Advancements in Diagnostic Technologies
Innovations in imaging techniques, such as cardiac MRI, echocardiography, and nuclear scintigraphy, enable earlier and more accurate detection of cardiac amyloidosis. These advancements are improving patient outcomes and driving market growth.
-
Development of Novel Therapies
The emergence of disease-modifying therapies, including monoclonal antibodies and RNA-targeted treatments, is expanding treatment options for cardiac amyloidosis patients, boosting market adoption.
-
Increasing Awareness Among Healthcare Professionals
Greater knowledge of cardiac amyloidosis symptoms and progression among cardiologists and other specialists leads to early intervention and increased use of diagnostics and therapies.
-
Aging Population
Cardiac amyloidosis primarily affects older adults, particularly those above 60 years. With the global geriatric population increasing, the number of patients at risk is growing, driving market demand.
-
Rising Focus on Personalized Medicine
Personalized approaches to managing cardiac amyloidosis, including tailored therapeutic regimens and monitoring strategies, are encouraging the development and adoption of advanced treatments.
-
Expansion of Healthcare Infrastructure in Emerging Markets
Improved access to cardiac care, diagnostic facilities, and specialty treatments in emerging regions is supporting market growth by increasing patient reach.
DelveInsight’s comprehensive report titled “Cardiac Amyloidosis Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of cardiac amyloidosis. The report presents historical and projected epidemiological data covering Total Prevalent Cases and Diagnosed Prevalent Cases of Cardiac Amyloidosis further segmented by Gender, Age, and Etiology. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in cardiac amyloidosis. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Cardiac Amyloidosis Overview
Amyloidosis refers to the extracellular deposition of fibrils that are composed of low molecular weight subunits of a variety of serum proteins. Amyloid can occur in a variety of organs, with the involvement of the heart, kidney, liver, and autonomic nervous system being most often responsible for the observed morbidity and mortality.
Cardiac amyloidosis is a disorder caused by amyloid fibril deposition in the extracellular space of the heart. Among the different types of amyloidosis, nearly all cases of clinical cardiac amyloidosis (>95%) are caused by light chain amyloidosis (AL) and transthyretin amyloidosis (ATTR).
Cardiac involvement in amyloidosis, termed cardiac amyloidosis or amyloid cardiomyopathy, typically presents with heart failure symptoms and signs with prominent features of right ventricular failure (peripheral congestion) including lower extremity oedema, jugular vein distention, hepatic congestion and ascites as well as dyspnoea.
Risk factors of cardiac amyloidosis include age older than 40, male gender, family history, and other diseases such as multiple myeloma, among others. Symptoms of cardiac amyloidosis include congestive heart failure, heart rhythm abnormalities such as lightheadedness, dizziness, palpitations shortness of breath, fatigue, and vascular disease, among others.
Cardiac Amyloidosis Diagnosis and Treatment Algorithm
Cardiac amyloidosis is an underdiagnosed disease entity. Its systemic manifestations and variety of symptoms based on etiological type make it under-recognized.
Cardiac amyloidosis diagnosis involves clinical evaluation, blood tests, imaging (echocardiography, MRI/CT), endomyocardial biopsy, and nuclear scans. Genetic testing may identify hereditary forms. A comprehensive, multidisciplinary approach is essential for accurate identification and timely intervention.
Treatment of cardiac amyloidosis requires a two-pronged approach. Some therapies aim to alleviate cardiac symptoms and complications, while others treat the underlying condition. For ATTR, stabilization of transthyretin with TTR stabilizers (Tafamidis) or liver protein alteration with TTR silencers (Patisiran/Inotersen) is used. AL is addressed with chemotherapy or stem cell transplant targeting abnormal plasma cells. Symptomatic relief includes diuretics, salt reduction, blood thinners, and, in severe cases, heart transplantation. This comprehensive approach addresses both the underlying condition and cardiac complications in managing cardiac amyloidosis.
Cardiac Amyloidosis Epidemiology
The epidemiology section on the cardiac amyloidosis market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of cardiac amyloidosis. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings from Cardiac Amyloidosis Epidemiological Analyses and Forecast
- During the analysis of cardiac amyloidosis, it was determined that the prevalence of the condition is approximately 20.1% in Spain.
- In the analysis, it was found that the majority of cases of cardiac amyloidosis were transthyretin-related amyloidosis (ATTR-CM), accounting for approximately 84.6% of the cases. A smaller proportion of cases were attributed to cardiac light-chain amyloidosis (AL-CM), representing approximately 2.2% of the total cases.
- The analysis revealed a significant gender difference in the prevalence of cardiac amyloidosis, with a notably higher prevalence observed in men compared to women. Specifically, the prevalence among men was approximately 60.1%.
- Secondary analysis unveiled a significant trend suggesting that the prevalence of cardiac amyloidosis increases with advancing age. Specifically, the prevalence was found to be 1% (1 out of 75 individuals) among those aged 40–49, 2% (2 out of 86) among those aged 50–59, 9% (8 out of 84) among those aged 60–69, 21% (13 out of 61) among those aged 70–79, and 26% (8 out of 31) among those aged 80 or older. This trend was statistically significant, with a p-value of less than 0.01.
- The epidemiology of cardiac amyloidosis is expected to change during the forecast period (2024-2034).
Cardiac Amyloidosis Market Outlook
The cardiac amyloidosis therapeutics market is further expected to increase by the major drivers, such as the rising prevalent population, technological advancements, and upcoming therapies in the forecast period [2024–2034].
In May 2019, the US FDA approved VYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) for the treatment of the cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization.
In March 2019, the Ministry of Labor Health and Welfare in Japan approved VYNDAQEL, under the SAKIGAKE designation, for patients with wild-type and variant forms of ATTR-CM. Regulatory submissions for the use of VYNDAQEL in patients with ATTR-CM have been submitted to the European Medicines Agency (EMA) and are under review.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the cardiac amyloidosis market in the 7MM is expected to change significantly during the study period 2020–2034.
Cardiac Amyloidosis Recent Developments
- In January 2025, Life Molecular Imaging secured fast-track designation from the U.S. FDA for its amyloid PET radiotracer, used to visualize cardiac amyloid light-chain (AL) and amyloid transthyretin-related (ATTR) amyloidosis.
Cardiac Amyloidosis Drug Chapters
Marketed Cardiac Amyloidosis Drugs
- VYNDAQEL and VYNDAMAX: Pfizer
VYNDAQEL and VYNDAMAX are two oral formulations of the first-in-class transthyretin stabilizer tafamidis, and the first and only medicines approved by the FDA to treat ATTR-CM. VYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) are oral transthyretin stabilizers that selectively bind to transthyretin, stabilizing the tetramer of the transthyretin transport protein and slowing the formation of amyloid that causes ATTR-CM.
VYNDAQEL was granted Orphan Drug Designation for ATTR-CM in both the EU and the US in 2012 and in Japan in 2018. In June 2017 and May 2018, respectively, the FDA granted VYNDAQEL Fast Track and Breakthrough Therapy designations for ATTR-CM. In November 2018, the FDA granted Priority Review for the new drug application (NDA) for VYNDAQEL.
Note: Detailed marketed therapies assessment will be provided in the final report...
Emerging Cardiac Amyloidosis Drugs
The cardiac amyloidosis market is expected to experience gradual changes, mainly due to the limited availability of emerging therapies in this area. Key market players, including Alnylam Pharmaceuticals, Alexion Pharmaceuticals, Eidos Therapeutics, Ionis Pharmaceuticals, AstraZeneca, and others have demonstrated a keen interest in this condition and are actively pursuing the development of potential treatments.
- Vutrisiran: Alnylam Pharmaceuticals
Vutrisiran is an investigational RNAi therapeutic in development for the treatment of transthyretin-mediated (ATTR) amyloidosis, which encompasses both hereditary ATTR (hATTR) amyloidosis and wild-type ATTR (wtATTR) amyloidosis. Vutrisiran inhibits the production of disease-causing transthyretin (TTR) protein by the liver, leading to a reduction in the level of TTR in the blood. Currently the drug is being evaluated in a Phase III study to evaluate Vutrisiran in patients with transthyretin amyloidosis with cardiomyopathy.
- Acoramidis: Alexion Pharmaceuticals/Eidos Therapeutics/Bridgebio
Acoramidis is an investigational, oral, small molecule. Alexion holds an exclusive license to develop and commercialize acoramidis in Japan. The drug is currently being evaluated in a Phase III study of ALXN2060 in Japanese participants with symptomatic ATTR-CM. In December 2023, BridgeBio submitted an NDA for acoramidis to the US FDA for the treatment of ATTR-CM.
-
Eplontersen: Ionis Pharmaceuticals/AstraZeneca
Eplontersen is a ligand-conjugated antisense (LICA) investigational medicine designed to reduce the production of transthyretin, or TTR protein, to treat all types of ATTR, a systemic, progressive, and fatal disease. Eplontersen is designed as a monthly self-administered subcutaneous injection to treat all types of ATTR. Currently, the drug is in Phase III of clinical development for the treatment of Transthyretin Amyloid Cardiomyopathy.
Note: Detailed emerging therapies assessment will be provided in the final report….
Cardiac Amyloidosis Market Segmentation
DelveInsight’s ‘Cardiac Amyloidosis – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future cardiac amyloidosis market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
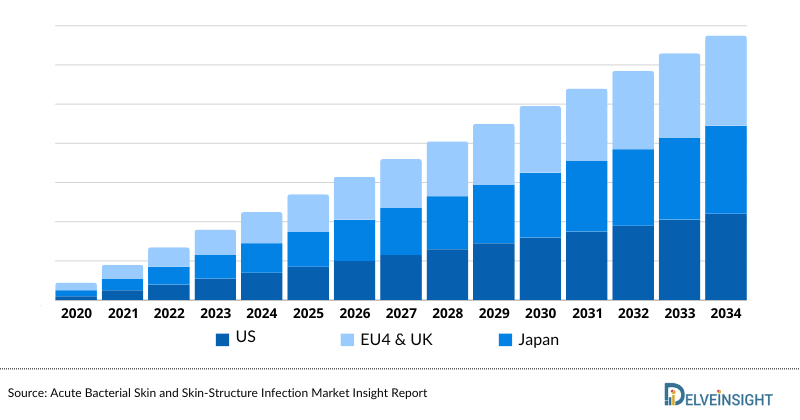
Cardiac Amyloidosis Market Size by Countries
The cardiac amyloidosis market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) cardiac amyloidosis market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
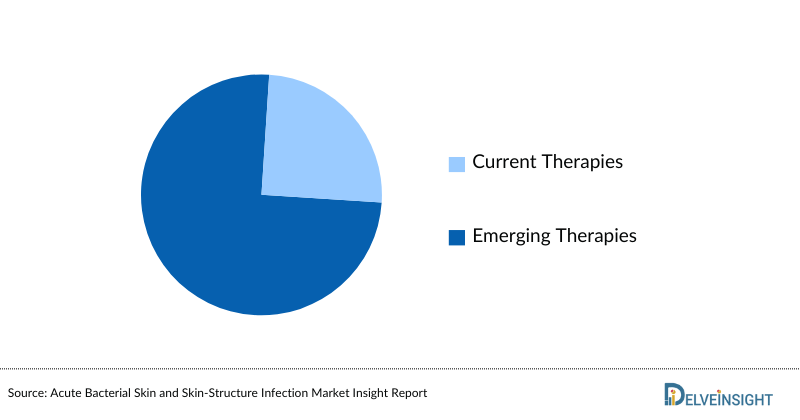
Cardiac Amyloidosis Market Size by Therapies
Cardiac Amyloidosis Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is Vutrisiran under the developmental pipeline of Alnylam Pharmaceuticals.
Note: Detailed market segment assessment will be provided in the final report….
Cardiac Amyloidosis Drugs Uptake
This section focuses on the sales uptake of potential cardiac amyloidosis drugs that have recently been launched or are anticipated to be launched in the cardiac amyloidosis market between 2020 and 2034. It estimates the market penetration of cardiac amyloidosis drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the cardiac amyloidosis market.
The emerging cardiac amyloidosis therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the cardiac amyloidosis market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Cardiac Amyloidosis...
Cardiac Amyloidosis Market Access and Reimbursement
DelveInsight’s ‘Cardiac Amyloidosis – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of cardiac amyloidosis.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Latest KOL Views on Cardiac Amyloidosis Market
To keep up with current cardiac amyloidosis market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the cardiac amyloidosis domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or cardiac amyloidosis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the cardiac amyloidosis unmet needs.
Cardiac Amyloidosis: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the American College of Cardiology, British Heart Foundation, European Society of Cardiology, National Amyloidosis Centre, University College London in the UK, Internal Medicine Department, Hospital Universitario Virgen Macarena in Spain, and Department of Medicine, Universidad de Sevilla in Spain, among others.
“The current market of Cardiac Amyloidosis lacks potential products that can prove to be effective for patients with Cardiac Amyloidosis.”
“Cardiac amyloidosis presents a significant health concern, with prevalence increasing notably with age. Early detection and tailored interventions are crucial for improving outcomes.”
Note: Detailed assessment of KOL Views will be provided in the full report on Cardiac Amyloidosis....
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the cardiac amyloidosis Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Cardiac Amyloidosis Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for cardiac amyloidosis. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, patent details, and other information for emerging cardiac amyloidosis therapies.
Cardiac Amyloidosis Report Insights
- Cardiac Amyloidosis Patient Population
- Therapeutic Approaches
- Cardiac Amyloidosis Pipeline Analysis
- Cardiac Amyloidosis Market Size and Trends
- Cardiac Amyloidosis Market Opportunities
- Impact of Upcoming Therapies
Cardiac Amyloidosis Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Cardiac Amyloidosis Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Cardiac Amyloidosis Market
- Cardiac Amyloidosis Drugs Uptake
Cardiac Amyloidosis Report Assessment
- Cardiac Amyloidosis Current Treatment Practices
- Unmet Needs
- Cardiac Amyloidosis Pipeline Product Profiles
- Cardiac Amyloidosis Market Attractiveness
Key Questions Answered in the Cardiac Amyloidosis Report
- How common is Cardiac Amyloidosis?
- What are the key findings of cardiac amyloidosis epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for cardiac amyloidosis?
- What are the disease risk, burden, and unmet needs of cardiac amyloidosis?
- At what CAGR is the cardiac amyloidosis market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the cardiac amyloidosis market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of cardiac amyloidosis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many companies are currently developing therapies for the treatment of cardiac amyloidosis?