Chronic Lower Back Pain Market
- Chronic Lower Back Pain is pain that persists for 12 weeks or longer, even after an initial injury or underlying cause of acute lower back pain. Low back pain is very common, and everyone must have faced this problem at one point.
- In 2023, the market size of CLBP was highest in the US among the 7MM accounting for approximately USD 4,218 million that is further expected to increase at a CAGR of 4%.
- The current treatment strategies mainly rely upon the use of opioids such as Xtampza ER (oxycodone), developed by Collegium Pharmaceutical to treat moderate-to-severe pain, and BELBUCA, developed by BioDelivery Sciences International, for treating low back pain as well as NSAIDs, Antidepressants, Anticonvulsants, and others. Opioids accounted for highest market share of ~2,963 million in 2023, for treatment of Chronic Lower Back Pain in the 7MM.
- CLBP approximately accounted for ~68 million diagnosed prevalent cases in 2023 in the 7MM, the treatment market of CLBP lacks approved therapy specific to CLBP treatment.
- The Chronic Lower Back Pain treatment market in the 7MM is driven by an aging population, rising prevalence due to sedentary lifestyles and stress, and advancements in treatments and diagnostics. Increased demand is driven by age-related spinal changes and the need for improved therapies and early interventions.
- Scilex Pharmaceuticals, Inc.’s SP-102 (SEMDEXA) is a non-opioid corticosteroid formulated as a viscous gel injection that is under development to treat lumbar radicular pain/sciatica, containing no neurotoxic preservatives, surfactants, solvents, or particulates. It is estimated to be launch by 2025 in the US. This emerging therapy have the potential to create a significant positive shift in the CLBP market size.
Request for unlocking the sample page of the "Chronic Lower Back Pain Treatment Market"
DelveInsight’s “Chronic Lower Back Pain Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Chronic Lower Back Pain, historical and forecasted epidemiology as well as the Chronic Lower Back Pain market trends in the United States, EU4, and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Chronic Lower Back Pain treatment market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Chronic Lower Back Pain market size from 2020 to 2034. The Report also covers current Chronic Lower Back Pain treatment market practices, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Chronic Lower Back Pain Treatment Market
Chronic low back pain (CLBP) is pain that persists for 12 weeks or longer, even after an initial injury or underlying cause of acute low back pain. Low back pain is widespread and likely to affect everyone at one point. The exact cause of lower back pain is not found yet. Long-term lower back pain (for more than 3 months) is called CLBP. CLBP is the second leading cause of disability worldwide, being a major welfare and economic problem. The prevalence of CLBP in adults has increased significantly in the last decade and is continuously increasing vividly in the aging population. CLBP has adverse physical and psychological repercussions. It creates a significant economic burden due to loss of function, loss of work productivity, treatment costs, and disability payments.
Chronic Lower Back Pain Diagnosis
CLBP is usually investigated by physician evaluation or by imaging studies. The primary goal of diagnosis is to characterize the pain as mechanical, primarily when history is obtained from a patient with CLBP and sciatica. Mechanical or activity-related spinal pain is most often aggravated by static loading of the spine (prolonged sitting or standing), long-lever activities (vacuuming or working with the arms elevated and away from the body), and levered postures (forward bending of the lumbar spine). Pain is reduced when multidirectional forces balance the spine (walking or constantly changing positions) and when the spine is unloaded. Patients with mechanical LBP often prefer to lie still in bed. In contrast, those with a vascular or visceral cause are often found writhing in pain, unable to find a comfortable position.
Further details related to diagnosis are provided in the report...
Chronic Lower Back Pain Treatment
For some patients, CLBP can be relieved by single-agent therapy, but as both nociceptive and neuropathic mechanisms are often present, combining agents with different mechanisms of action is a rational approach. Analgesics, anti-inflammatory drugs, muscle relaxants, and other medications can be used to help control chronic back pain. However, most have unwanted side effects and are not intended for prolonged use. Opioid medication should not be used without a physician’s recommendation.
Nonopioid analgesics such as acetaminophen (APAP), nonsteroidal anti-inflammatory drugs (NSAIDs), or cyclooxygenase-2 (COX-2) inhibitors are used for initial management of low back pain. The use of muscle relaxants should be avoided in patients with CLBP. Treatment of mild CLBP often begins with the use of NSAIDs and mild nonopioid analgesics, while more potent opioid analgesics are reserved for pain of moderate-to-severe intensity.
Chronic Lower Back Pain Epidemiology
As the market is derived using a patient-based model, the Chronic Lower Back Pain epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Prevalent Cases of CLBP, Diagnosed Prevalent Cases of CLBP, Gender-specific Diagnosed Prevalent Cases of CLBP, and Age-specific Diagnosed Prevalent Cases of CLBP, in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
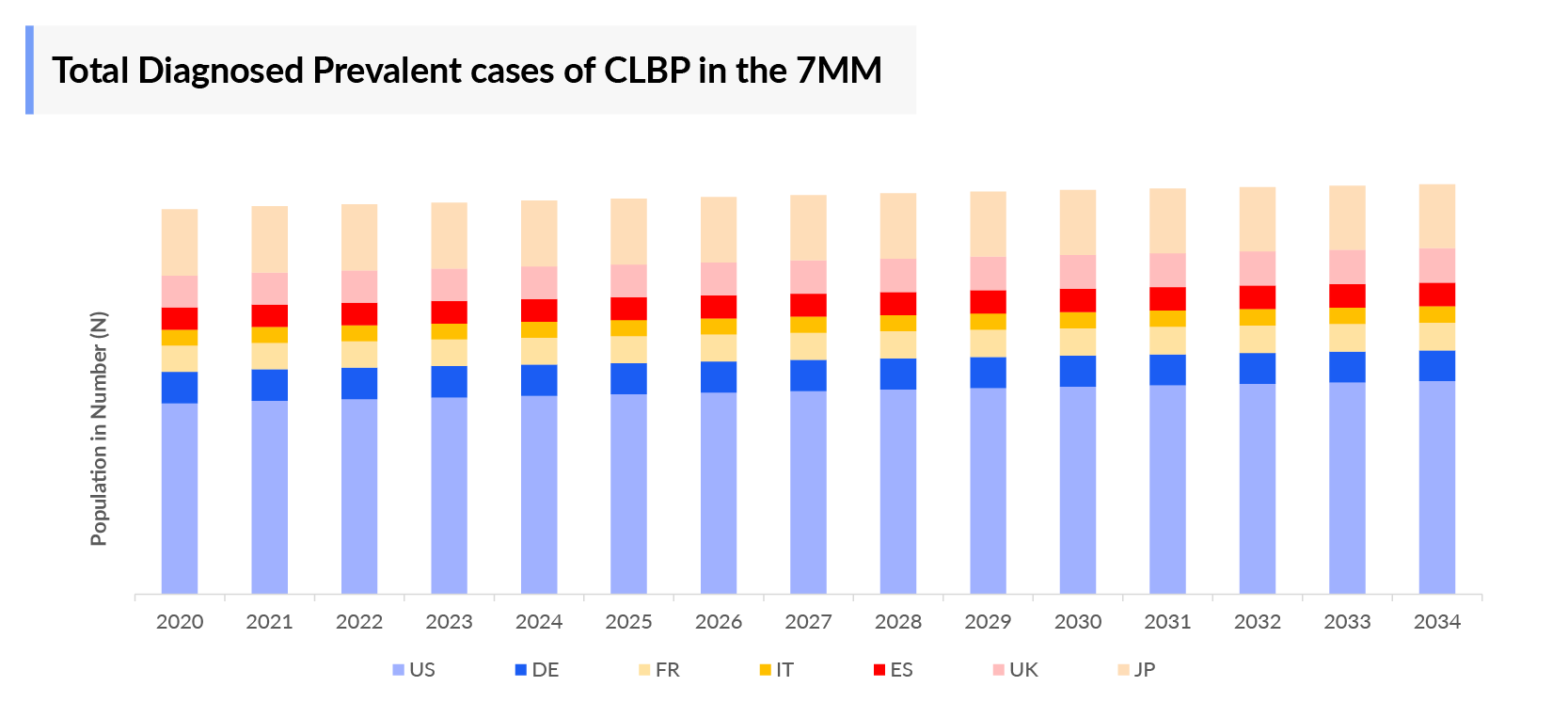
- The total diagnosed prevalent cases of CLBP in the United States were around 31 million cases in 2023.
- The United States contributed to the largest diagnosed prevalent population of CLBP, acquiring ~45% of the 7MM in 2023. Whereas, EU4 and the UK, and Japan accounted for around 40% and 15% of the total population share, respectively, in 2023.
- Among the EU4 countries, Spain accounted for the largest number of Prevalent CLBP (8.6 million) cases followed by Germany (6.3 million), whereas France accounted for the lowest number of cases (5 million) in 2023.
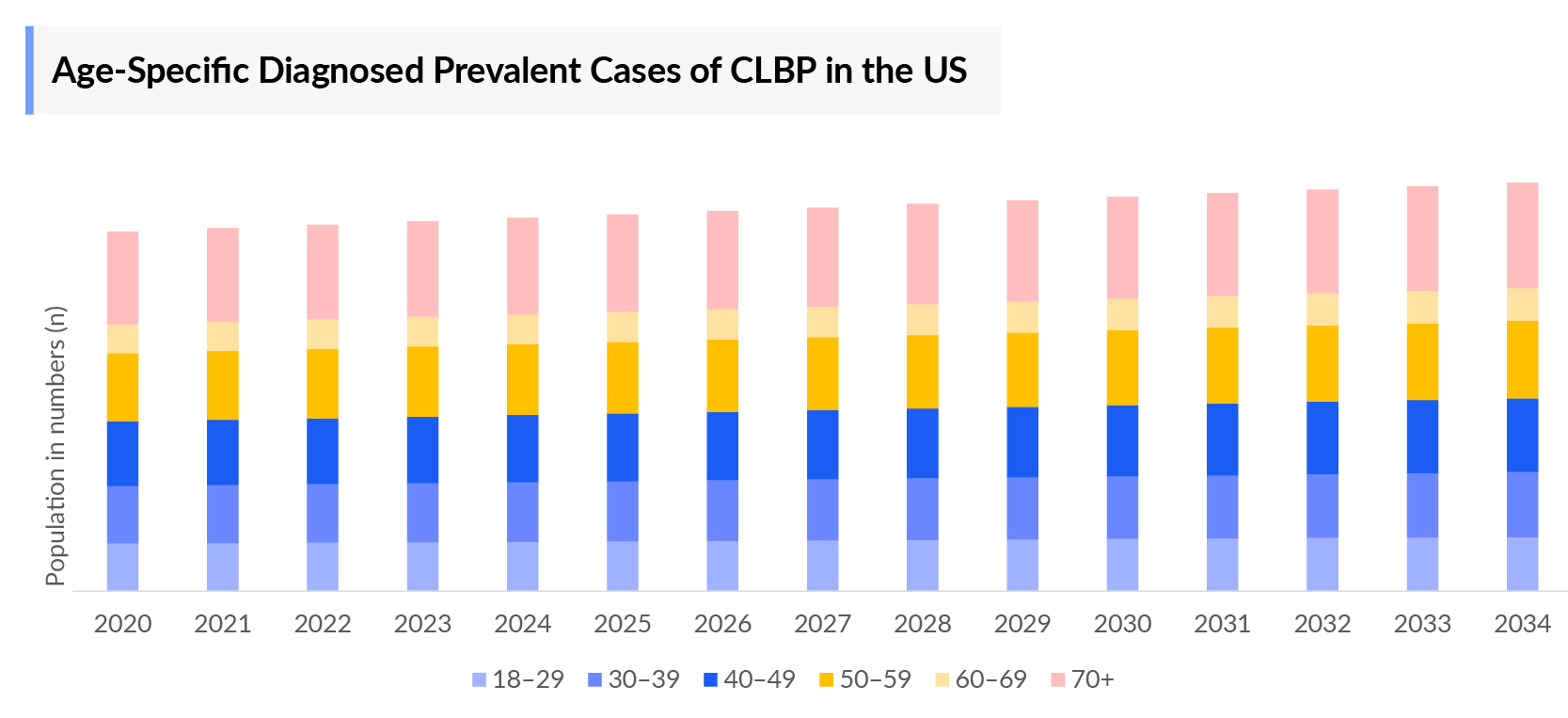
- In 2023, it was estimated that there were around 8 million diagnosed cases in the age group of 70 years and older in the US.
- According to DelveInsight estimates, in 2023 there were approximately 5.5 million diagnosed prevalent cases of chronic low back pain (CLBP) among males and about 4.5 million cases among females in Japan.
- DelveInsight analysis of the age-specific data in the US revealed that the highest number of people affected with CLBP were found in the age group of 70+ years, while people who belonged to the age group 60–69 years were the least affected.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Chronic Lower Back Pain Prevalence
Chronic Lower Back Pain Recent Developments
- In January 2025, Pacira BioSciences, Inc. received FDA clearance for a new Smart Tip designed to access medial branch nerves for managing chronic low back pain. The ioveraº system offers a drug-free solution, using cryoneurolysis to apply cold therapy to targeted nerves, temporarily blocking pain signals.
Chronic Lower Back Pain Drug Chapters
The drug chapter segment of the Chronic Lower Back Pain drugs market report encloses a detailed analysis of Chronic Lower Back Pain off-label drugs and late-stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Chronic Lower Back Pain clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Chronic Lower Back Pain news and press releases.
Chronic Lower Back Pain Marketed Drugs
XTAMPZA ER (oxycodone): Collegium Pharmaceutical
XTAMPZA ER (oxycodone) is a semisynthetic opioid. It is currently indicated as an immediate-release product for moderate-to-severe pain and as an extended-release product for chronic moderate-to-severe pain requiring continuous opioid analgesics for an extended period. It is used to manage acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Oxycodone is a highly selective full agonist of the µ-opioid receptor (MOR), with low affinity for the d-opioid receptor (DOR) and ?-opioid receptor (KOR). Oxycodone and its active metabolites can selectively bind to the µ-opioid receptor and the kappa and delta-opioid receptors in the central nervous system and periphery and induce a G protein-coupled receptor signaling pathway.
In November 2015, the FDA granted tentative approval to the NDA for XTAMPZA for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
Chronic Lower Back Pain Emerging Drugs
- Rexlemestrocel-L (MPC-06-ID): Mesoblast Limited/Grünenthal
Rexlemestrocel-L, also known as MPC-06-ID, is a Mesoblast’s proprietary allogeneic mesenchymal precursor cell (MPC) product candidate, currently in the late stage of development for the treatment of CLBP caused by disc degeneration. It is being developed for patients who have exhausted conservative treatment options, may have failed epidural steroid injections, and have no further treatment option other than invasive and costly surgical interventions.
In February 2023, the FDA OTAT granted RMAT designation for rexlemestrocel-L in the treatment of CLBP associated with disc degeneration, in combination with HA as a delivery agent for injection into the lumbar disc.
Early in 2021, Mesoblast published results from the Phase III trial MSBDR003 that was carried out in the US and Australia. The trial provided a number of important findings, including a significant and long-lasting treatment effect on pain relief. However, it did not achieve its primary outcome measure between the treatment groups.
After having analysed the data obtained through this trial, Mesoblast is conducting another confirmatory trial in the US, and to design this trial to support potential parallel product approvals in both the US and Europe.
The latest Phase III trial to evaluate the efficacy of rexlemestrocel-L+HA compared to control in reducing low back pain at 12 months post-treatment and safety of a single injection of rexlemestrocel-L+HA injected into a lumbar intervertebral disc compared to control through 12 months post-treatment is anticipated to get completed by October 2026.
- Semdexa (SP-102): Scilex Holding Company
Semdexa (SP-102, dexamethasone sodium phosphate) is a non-opioid corticosteroid formulated as a viscous gel injection that is under development to treat lumbar radicular pain/sciatica, containing no neurotoxic preservatives, surfactants, solvents, or particulates. Because of these properties, the drug is expected to have a better safety profile than commonly used off-label injected corticosteroids. SP-102 has an ingredient that is a widely used corticosteroid solution.
In January 2018, SP-102 received the Fast Track designation from the US FDA to treat lumbar radicular pain.
With a patent extending until 2036, SP-102 achieved its Phase III endpoints in the first half of 2022. In the second half of 2023, the FDA agreed on the NDA pathway for the drug. Currently, in 2024, the final Phase III safety trial is being completed to finalize the NDA package.
In July 2024, Semnur Pharmaceuticals, Inc., a subsidiary of Scilex Holding Company, and Denali Capital Acquisition Corp. (Nasdaq: DECA) signed a letter of intent for a proposed business combination. This deal values Semnur at up to USD 2.0 billion, subject to adjustments, with expected cash on hand of up to USD 40 million at closing. The merger aims to create a publicly traded biopharma company and secure funding for Semnur's development of SP-102 (SEMDEXA), a Phase III non-opioid treatment for sciatica with FDA Fast Track status.
 Chronic Lower Back Pain Market Outlook
Chronic Lower Back Pain Market Outlook
CLBP is a complex process comparable to how each patient has a very individualized disease process the treatment regimen is similarly individualized. There are several different medication classes, each with an exclusive mechanism of action that can help the practitioner target a specific aspect of a patient's pain. Moreover, patient-specific factors are considered when developing a regimen to ensure adherence and improve outcomes.
Most (80–90%) of low back pain cases stem from a mechanical origin, such as degenerative disc or joint disease, vertebral fractures, and deformities. Neurogenic, inflammatory, and other less common causes comprise the remaining etiologies. The initial pharmacological agents chosen should align with the underlying etiology. However, as pain transitions to a chronic state, a more comprehensive approach is often necessary due to the reduced efficacy of targeted treatments.
However, if the cause of low back pain is inflammatory, targeted therapy includes treatment with anti-inflammatory agents. This refers to the early use of nonsteroidal anti-inflammatories (NSAIDs) and treatment with corticosteroids or disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis or ankylosing spondylitis.
To manage the symptoms and to reduce the extent of pain, certain drugs, such as Xtampza (Collegium Pharmaceutical) and BELBUCA (buprenorphine HCL buccal film; BioDelivery Sciences International), have been approved by the US FDA for the management of severe pain enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
Few new agents are being developed and tested as potential treatments for CLBP; the emerging drugs include Rexlemestrocel-L (MPC-06-ID) by Mesoblast Limited/Grünenthal, Cebranopadol (TRN-228) by Tris Pharma, and others.
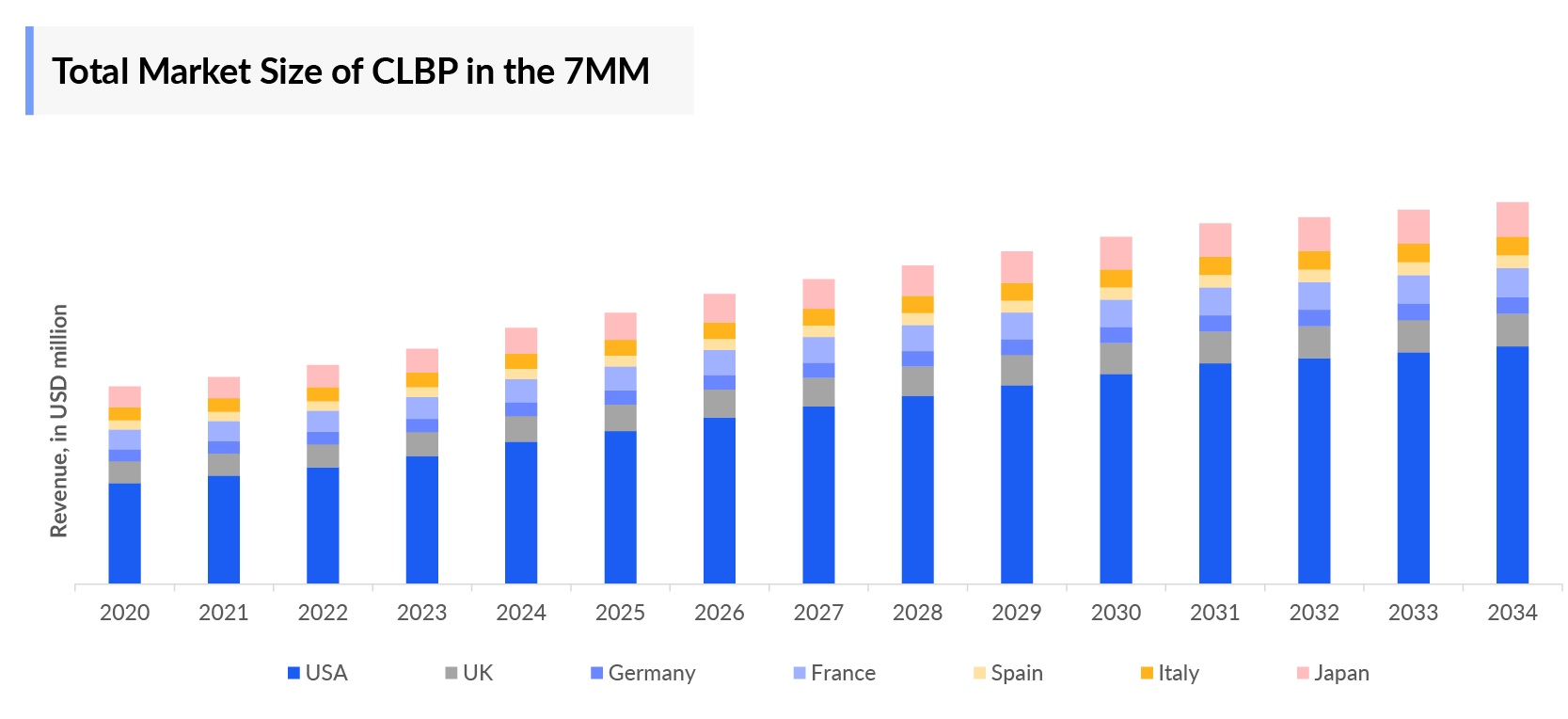
- The Chronic Lower Back Pain Therapeutics Market Size in the 7MM was approximately USD 6,071 million in 2023.
- The Chronic Lower Back Pain Market Size in the 7MM will increase at a CAGR of 3.7% due to increasing awareness of the disease, better diagnosis, and the launch of the emerging therapy.
- The United States accounted for the highest Chronic Lower Back Pain Therapeutics Market Size approximately 69% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the European countries, Spain had the highest Chronic Lower Back Pain market size with nearly USD 312 million in 2023, while Italy had the lowest market size of Chronic Lower Back Pain with ~USD 158 million in 2023.
- The Chronic Lower Back Pain Therapeutics Market Size in Japan was estimated to be ~USD 866 million in 2023, which accounts for 14% of the total 7MM market.
- With the expected launch of upcoming therapies, such as SEMDEXA (SP-102) the total Chronic Lower Back Pain Treatment Market Size is expected to show change in the upcoming years.
Chronic Lower Back Pain Drugs Uptake
This section focuses on the uptake rate of potential Chronic Lower Back Pain drugs expected to launch in the market during 2020–2034. For example, SEMDEXA (SP-102) in the US is expected to be launched by 2025 with a peak share of 1%. SEMDEXA (SP-102) is anticipated to take 7 years to peak with a slow-medium uptake.
Chronic Lower Back Pain Pipeline Development Activities
The Chronic Lower Back Pain therapeutics market report provides insights into different Chronic Lower Back Pain clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Chronic Lower Back Pain Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Chronic Lower Back Pain therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chronic Lower Back Pain emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Chronic Lower Back Pain Treatment Drugs
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Chronic Lower Back Pain evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Princeton Spine & Joint Center, USA; Department of Anesthesiology, University of Michigan, USA; University Hospital Giessen, Germany; University of Salerno, Italy; Pain Unit, University Hospital Puerta Del Mar, Cádiz, Spain; University of Southampton, Southampton General Hospital, United Kingdom; Saitama Medical University, Japan; Pain Clinic and Palliative Care, University Hospital, Kyoto Prefectural University of Medicine, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Chronic Lower Back Pain market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Chronic Lower Back Pain Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chronic Lower Back Pain Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Chronic Lower Back Pain drugs market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Chronic Lower Back Pain Therapeutics Market Report Scope
- The Chronic Lower Back Pain therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Chronic Lower Back Pain treatment market landscape.
- A detailed review of the Chronic Lower Back Pain drugs market, historical and forecasted Chronic Lower Back Pain therapeutics market size, Chronic Lower Back Pain drugs market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Chronic Lower Back Pain therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Chronic Lower Back Pain Drugs Market.
Chronic Lower Back Pain Drugs Market Report Insights
- Patient-based Chronic Lower Back Pain Market Forecasting
- Chronic Lower Back Pain Therapeutic Approaches
- Chronic Lower Back Pain Pipeline Analysis
- Chronic Lower Back Pain Market Size and Trends
- Existing and Future Chronic Lower Back Pain Therapeutics Market Opportunities
Chronic Lower Back Pain Therapeutics Market Report Key Strengths
- 11 years Chronic Lower Back Pain Market Forecast
- The 7MM Coverage
- Chronic Lower Back Pain Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Chronic Lower Back Pain Drugs Uptake
- Key Chronic Lower Back Pain Market Forecast Assumptions
Chronic Lower Back Pain Therapeutics Market Report Assessment
- Current Chronic Lower Back Pain Treatment Market Practices
- Chronic Lower Back Pain Unmet Needs
- Chronic Lower Back Pain Pipeline Product Profiles
- Chronic Lower Back Pain Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Chronic Lower Back Pain Market Drivers
- Chronic Lower Back Pain Market Barriers
Key Questions Answered
Chronic Lower Back Pain Treatment Market Insights
- What was the Chronic Lower Back Pain Drugs Market Share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Chronic Lower Back Pain market size as well as Chronic Lower Back Pain treatment market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Chronic Lower Back Pain market size during the forecast period (2024–2034)?
- At what CAGR, the Chronic Lower Back Pain treatment market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Chronic Lower Back Pain market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Chronic Lower Back Pain drugs market growth till 2034 and what will be the resultant Chronic Lower Back Pain treatment market size in the year 2034?
- How would the Chronic Lower Back Pain market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Chronic Lower Back Pain Epidemiology Insights
- What are the disease risk, burden, and Chronic Lower Back Pain unmet needs?
- What is the historical Chronic Lower Back Pain patient population in the United States, EU4 (Germany, France, Italy, Spain) and the UK, and Japan?
- What would be the forecasted patient population of Chronic Lower Back Pain at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Chronic Lower Back Pain?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Chronic Lower Back Pain during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Chronic Lower Back Pain Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Chronic Lower Back Pain along with the approved therapy?
- What are the current treatment guidelines for the treatment of Chronic Lower Back Pain in the US, Europe, And Japan?
- What are the Chronic Lower Back Pain-marketed drugs and their Chronic Lower Back Pain MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many companies are developing therapies for the treatment of Chronic Lower Back Pain?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Chronic Lower Back Pain?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to the Chronic Lower Back Pain therapies?
- What are the recent therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for Chronic Lower Back Pain and their status?
- What are the key designations that have been granted for the emerging therapies for Chronic Lower Back Pain?
- What are the 7MM historical and forecasted Chronic Lower Back Pain Drugs Market?
Reasons to Buy
- The Chronic Lower Back Pain Drugs Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Chronic Lower Back Pain Treatment Market.
- Insights on patient burden/disease Chronic Lower Back Pain prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Chronic Lower Back Pain Treatment Market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Chronic Lower Back Pain Treatment Market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Chronic Lower Back Pain Treatment Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles
-Market.png&w=256&q=75)