Congenital Hyperinsulinism Market
- The Congenital HyperInsulinism Treatment Market Growth is expected to be mainly driven by the entry of novel therapies with better Congenital HyperInsulinism clinical trials profiles, an increase in market penetration of advanced therapies, an upsurge in research and development, an enriched understanding of the disease, and imminent launches of the drugs.
- Congenital Hyperinsulinism (CHI) is a rare disease that causes newborns and children to have low blood sugar due to abnormal insulin release. It is the most common cause of persistent hypoglycemia in infants and children.
- The United States accounts for the largest Congenital HyperInsulinism Market Share (around 40%), in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Only a limited number of Congenital HyperInsulinism Drugs are approved for the Congenital HyperInsulinism treatment, and several pharmacological options are used off-label. The first-line therapy for CHI is the adenosine triphosphate-sensitive potassium (KATP) channel activator
- PROGLYCEM (diazoxide) is the only therapy for CHI, approved by the FDA and the EMA.
- Various new Congenital HyperInsulinism Therapies are currently in development. Some of the most prominent ones include dasiglucagon (Zealand Pharma), and RZ358 (Rezolute), among others.
- According to Delveinsight’s estimates, RZ358 is expected to garner the highest Congenital HyperInsulinism drugs market share by 2034, in the seven major markets.
- Zealand Pharma intends to resubmit its NDA to the FDA in the first half of 2024 for ZEGALOGUE (dasiglucagon), investigated to treat pediatric hypoglycemia in CHI patients, following a 2023 Complete Response Letter related to manufacturing facility issues.
- As per DelveInsight's estimates, the EU4 and the UK exhibited the highest Congenital HyperInsulinism Diagnosed Prevalent Cases compared to the US and Japan. Together, they represented around 70% of the total cases of CHI among the 7MM in 2023
- In 2023, more than 90% of the total treated cases were for first-line treatment in the 7MM.
Request for Unlocking the Sample Page of the "Congenital HyperInsulinism Treatment Market"
DelveInsight's “Congenital Hyperinsulinism Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of congenital hyperinsulinism, historical and forecasted epidemiology as well as the congenital hyperinsulinism treatment market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Congenital Hyperinsulinism Treatment Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM congenital hyperinsulinism market size from 2020 to 2034. The report also covers current congenital hyperinsulinism treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the Congenital HyperInsulinism makret potential.
Congenital Hyperinsulinism Treatment Market Understanding and Algorithm
CHI is characterized by inappropriate and unregulated insulin secretion from the β cells of the pancreas. In CHI, the β cells release insulin inappropriately, and insulin secretion is not regulated by the blood glucose level (as occurs normally). The action of insulin causes hyperinsulinemic hypoglycemia. About 60% of babies with CHI develop hypoglycemia during the first month of life, and an additional 30% will be diagnosed later in the first year and the remainder after that. Most of the time, congenital hyperinsulinism is caused by mutations in the genes that regulate insulin. Mutations in different key genes (ABCC8, KCNJ11, GLUD1, GCK, HADH, SLC16A1, UCP2, HNF4A, HNF1A, HK1, PGM1, and PMM2) that are involved in the regulation of insulin secretion from pancreatic β cells have been described to be responsible for the underlying molecular mechanisms leading to congenital hyperinsulinism.
Diagnosing hyperinsulinism can be challenging due to fluctuating insulin levels. Hypoglycemia during glucose infusion and low levels of free fatty acids and ketones suggest excess insulin. The glucagon stimulation test and genetic testing help differentiate between focal and diffuse disease. Localization of focal lesions is achieved using specialized imaging like the 18-F-DOPA PET scan.
The congenital hyperinsulinism treatment market report provides an overview of congenital hyperinsulinism pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Congenital Hyperinsulinism Treatment
There are two options for treating congenital HI: Medical therapy and surgical intervention. About 50% of children respond to medical therapy, while the other half require surgery for a partial or near total pancreatectomy
Medical therapy for the management of infants and children with CHI is limited as, till now, only one drug, PROGLYCEM (diazoxide), has been approved by the FDA and EMA to treat CHI. Other drugs, including somatostatin analogs, such as octreotide and lanreotide, sirolimus, nifedipine, and glucagon, are being used as off-label therapy.
Congenital Hyperinsulinism Epidemiology
The congenital hyperinsulinism epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The congenital hyperinsulinism epidemiology is segmented with detailed insights into Total diagnosed prevalent Cases of Congenital Hyperinsulinism (CHI), Type-specific diagnosed prevalence based on histological presentation, Mutation specific diagnosed prevalence of Congenital Hyperinsulinism, and Total Treated Cases of Congenital Hyperinsulinism.
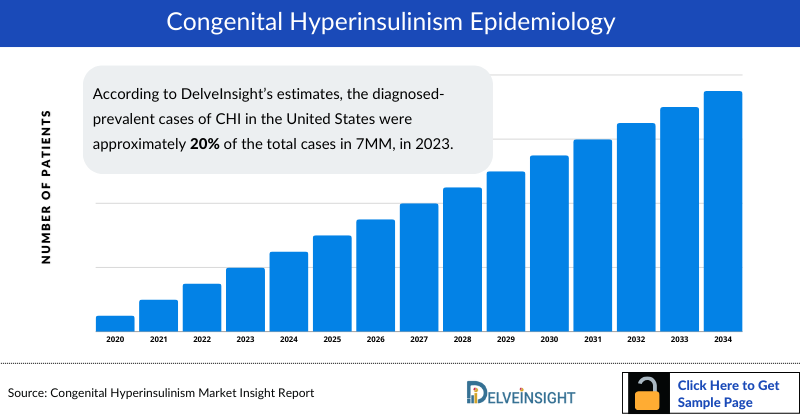
- According to DelveInsight's estimates, the Congenital HyperInsulinism Diagnosed Prevalent Cases in the United States were approximately 20% of the total cases in 7MM, in 2023.
- DelveInsight’s consultant estimates that the maximum number of cases belonged to the diffuse type of CHI. Approximately, 75% of CHI cases were of diffuse type in the United States in 2023.
- Based on Mutation-specific prevalence, it has been observed that ABCC8 and KCNJ11 are more common than other mutations in CHI patients.
- Among the EU4 and the UK, Germany had the highest prevalent population of CHI cases, followed by France.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Congenital HyperInsulinism Prevalence
Congenital Hyperinsulinism Recent Developments
- In May 2025, Rezolute, Inc. announced that the FDA granted Breakthrough Therapy Designation (BTD) to its investigational therapy, ersodetug, for the treatment of hypoglycemia caused by tumor-induced hyperinsulinism (HI).
- In January 2025, Handok's affiliate, Rezolute, announced that the FDA granted Breakthrough Therapy Designation to its treatment, RZ358 (ersodetug), for hypoglycemia caused by congenital hyperinsulinism.
Congenital Hyperinsulinism Drugs Market Chapters
The drug chapter segment of the congenital hyperinsulinism drugs market report encloses a detailed analysis of congenital hyperinsulinism marketed drugs and late-stage (Phase III and Phase II) Congenital HyperInsulinism pipeline drugs analysis. It also deep dives into the congenital hyperinsulinism clinical trials details, recent and expected market approvals, patent details, the latest Congenital HyperInsulinism news, and recent deals and Congenital HyperInsulinism collaborations.
Marketed Congenital HyperInsulinism Drugs
There is only one approved therapy for CHI in the market, namely, PROGLYCEM (diazoxide) - a non-diuretic benzothiadiazine derivative used for the management of symptomatic hypoglycemia. PROGLYCEM capsules and suspension are manufactured by IVAX Pharmaceuticals and Teva Pharmaceuticals, respectively; the suspension was manufactured for Gate Pharmaceuticals, a division of Teva Pharmaceuticals. In 2015, the FDA issued a warning for PROGLYCEM after reports of pulmonary hypertension in infants and newborns treated with the drug for low blood sugar. In December 2019, e5 Pharma launched the first FDA-approved generic of diazoxide. Additionally, several generic versions of this drug are also available in the market now.
Emerging Congenital HyperInsulinism Drugs
- ZEGALOGUE (dasiglucagon): Zealand Pharma
Dasiglucagon is a next-generation glucagon analog that is stable in an aqueous solution and is thus suitable for chronic pump use.Zealand expects to resubmit the New Drug Application (NDA) to the US Food and Drug Administration (FDA) for the prevention and treatment of hypoglycemia in pediatric patients seven days of age and older with CHI for up to three weeks of dosing, in the first half of 2024. The resubmission is in response to a Complete Response Letter issued by the FDA in December 2023 related to deficiencies identified at a third-party manufacturing facility that are not specific to dasiglucagon.
- RZ 358: Rezolute
RZ358, the lead asset, and the program is a monoclonal antibody (mAb) for treating hypoglycemia caused by CHI. RZ358 acts as a negative allosteric modulator of insulin at its receptor on insulin-dependent target tissues and is uniquely suited as a potential universal treatment for all forms of CHI. In December 2023 Rezolute announced the initiation of sunRIZE, a pivotal Phase III Congenital HyperInsulinism clinical trials of RZ358 in patients with congenital hyperinsulinism (cHI). Recently, in January 2024, the UK MHRA awarded the innovative medicine designation, the Innovation Passport, to RZ358 for the treatment of hypoglycemia due to congenital hyperinsulinism. In June 2020, the US FDA granted rare pediatric disease designation (RPDD) to RZ 358 for the treatment of CHI, in July 2016, EMA granted orphan drug designation (ODD) to RZ 358 for the treatment of CHI, and earlier in June 2015, an ODD was granted to RZ 358 by the US FDA for the treatment of congenital hyperinsulinemia.
|
Drugs |
Company |
Congenital HyperInsulinism MOA |
ROA |
Phase |
|
ZEGALOGUE (dasiglucagon) |
Zealand Pharma |
Glucagon receptor agonist |
Subcutaneous |
Registration |
|
RZ358 |
Rezolute |
Insulin receptor antagonists |
Intravenous |
III |
Note: Detailed emerging therapies assessment will be provided in the final report.
Congenital Hyperinsulinism Market Outlook
- Key Congenital HyperInsulinism Companies such as Zealand Pharma, Rezolute, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the congenital hyperinsulinism treatment.
- The United States represents approximately 40% of the Congenital HyperInsulinism Market Size surpassing EU4, the UK, and Japan.
- During the forecast period (2024–2034), Congenital HyperInsulinism Market Size is anticipated to be propelled by pipeline candidates such as RZ 358 (Rezolute), dasiglucagon (Zealand Pharma), and others.
- Germany accounts for the second highest Congenital HyperInsulinism Market Size in the 7MM during the forecast period 2024–2034.
- The Congenital HyperInsulinism therapeutics Market Size is expected to increase during the forecast period, i.e., 2024–2034, due to rising incidence rates, advancements in treatment options, improved diagnosis, and increased focus on rare diseases, supported by regulatory incentives driving innovation and investment.
Congenital Hyperinsulinism Drugs Uptake
This section focuses on the uptake rate of potential Congenital HyperInsulinism drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Congenital Hyperinsulinism Pipeline Development Activities
The Congenital HyperInsulinism therapeutics market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Congenital HyperInsulinism Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Congenital HyperInsulinism therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging congenital hyperinsulinism drugs.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Congenital HyperInsulinism Treatment Drugs
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Children's Hospital of Philadelphia, Ludwig Maximilian University of Munich, National Center for Child Health and Development, etc., were contacted. Their opinion helps understand and validate current and emerging congenital hyperinsulinism treatment patterns. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
Region |
KOL Views |
|
United States |
“From asymptomatic to severe hypoglycemia episodes that don't respond to medical therapy, the clinical characteristics can vary. In monogenic variants of CHI, a total of 14 genes have been linked up until recently. The most common and most severe monogenic form is caused by inactivating mutations of two subunits (SUR1 or Kir6.2) of the β-cell plasma membrane K+ATP channel encoded by ABCC8 and KCNJ11 genes, respectively. These patients can be separated into diazoxide-responsive and -nonresponsive instances for clinical care and prognostication. However, molecular testing is crucial for genetic counselling in both of these populations as well as for the treatment of patients who are not responding to diazoxide.” |
|
Japan |
“CHI is an ultra-rare disease of neonates and infants, and there are few systematic Congenital HyperInsulinism clinical trials. Octreotide has not been approved for the desired efficacy in any country in the world. It is widely used as a second-line drug for diazoxide-refractory cases in medical settings both in Japan and overseas and is also classified as a conventional second-line treatment.” |
Congenital HyperInsulinism Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Congenital HyperInsulinism Treatment Market Access and Reimbursement
Despite being a rare disease, CHI is associated with substantial annual costs to the NHS and Personal Social Services. The average annual cost of CHI to the NHS per patient per year was approximately EUR 2124 and the total annual cost of illness (COI) to the NHS was approximately EUR 3,408,398 per year. CHI patients with diffuse disease who were surgically managed incurred the greatest total annual costs compared with other treatment groups. Total annual costs attributable to this treatment group were approximately EUR 2,025,540, representing 59% of the total cost of CHI to the NHS per year.
The Congenital HyperInsulinism treatment market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Congenital HyperInsulinism Treatment Market Report Scope
- The Congenital HyperInsulinism therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the Congenital HyperInsulinism epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Congenital HyperInsulinism treatment market landscape.
- A detailed review of the congenital hyperinsulinism treatment market, historical and forecasted Congenital HyperInsulinism market size, Congenital HyperInsulinism drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Congenital HyperInsulinism treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM congenital hyperinsulinism drugs market.
Congenital Hyperinsulinism Treatment Market Report Insights
- Patient-based Congenital HyperInsulinism Market Forecasting
- Therapeutic Approaches
- Congenital Hyperinsulinism Pipeline Drugs Analysis
- Congenital Hyperinsulinism Market Size and Trends
- Existing and Future Congenital HyperInsulinism Drugs Market Opportunity
Congenital Hyperinsulinism Therapeutics Market Report Key Strengths
- 11 -year Congenital HyperInsulinism Market Forecast
- 7MM Coverage
- Congenital Hyperinsulinism Epidemiology Segmentation
- Inclusion of Country Specific Treatment Guidelines
- KOL’s Feedback On Approved and Emerging Therapies
- Key Cross Competition
- Conjoint Analysis
- Congenital HyperInsulinism Drugs Uptake
- Key Congenital HyperInsulinism Market Forecast Assumptions
Congenital Hyperinsulinism Treatment Market Report Assessment
- Current Congenital HyperInsulinism Treatment Market Practices
- Congenital HyperInsulinism Unmet Needs
- Congenital HyperInsulinism Pipeline Drugs Analysis Profiles
- Congenital HyperInsulinism Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM congenital hyperinsulinism treatment market?
- What was the congenital hyperinsulinism treatment market size, the Congenital HyperInsulinism market size by therapies, Congenital HyperInsulinism drugs market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of congenital hyperinsulinism?
- How many Congenital HyperInsulinism Companies are developing therapies for the treatment of congenital hyperinsulinism?
- What are the recent novel therapies, targets, Congenital HyperInsulinism mechanism of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The Congenital HyperInsulinism therapeutics market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the congenital hyperinsulinism drugs market.
- Insights on patient burden/disease Congenital HyperInsulinism prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Congenital HyperInsulinism drugs market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Congenital HyperInsulinism drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Congenital HyperInsulinism Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles




-pipeline.png&w=256&q=75)


