Congestive Heart Failure Market
- In 2023, the total Congestive Heart Failure Market Size was around USD 6,900 million in the 7MM—the increasing prevalence of the disease is expected to fuel the market during 2024–2034. According to the Heart Disease and Stroke Statistics-2023 updated report, the prevalence of heart failure continues to rise over time with the aging of the population, improved treatment of and survival with ischemic heart disease, and the availability of effective evidence-based therapies prolonging life in patients with heart failure.
- In 2023, the United States accounted for the largest number of prevalent cases of heart failure among the 7MM.
- Off-label therapies and generics occupy the major share of the current Congestive Heart Failure Market as they are cheaper and more easily accessible to patients. Generics of olmesartan, eplerenone, furosemide, and candesartan cilexetil are easily available in the market. The presence of generics and off-label therapies is a major barrier to newly developed drugs and hinders market growth.
- The increasing trend of existing key Congestive Heart Failure Companies such as AstraZeneca (FORXIGA), Novartis (ENTRESTO), Eli Lilly, and Boehringer Ingelheim (JARDIANCE), targeting on the label expansion and gaining approval regardless of the patient’s left ventricular ejection fraction status to cater to the wider range of population is likely to propel the Congestive Heart Failure drugs market.
- FUROSCIX’s FDA approval is a game-changer in treating heart failure, allowing certain patients to avoid costly hospitalizations altogether and simply self-administer treatment at home. The potential for cost savings—without sacrificing patient well-being in any way—will be too great for providers.
Request for Unlocking the Sample Page of the "Congestive Heart Failure Treatment Market"
DelveInsight’s “Congestive Heart Failure Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Congestive Heart Failure, historical and forecasted epidemiology as well as the Congestive Heart Failure market trends in the United States, EU4 (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Congestive Heart Failure Treatment Market Report provides current treatment practices, emerging drugs, Congestive Heart Failure market share of individual therapies, and current and forecasted Congestive Heart Failure market size from 2020 to 2034, segmented by seven major markets. The report also covers current Congestive Heart Failure treatment market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Congestive Heart Failure Therapeutics Market |
|
|
Congestive Heart Failures Treatment Market Size | |
|
Congestive Heart Failure Companies |
|
|
Congestive Heart Failure Epidemiology |
|
Congestive Heart Failure Treatment Market
DelveInsight’s Congestive Heart Failure treatment market report gives a thorough understanding of Congestive Heart Failure by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis, and treatment. Congestive Heart Failure commonly referred to as heart failure is a chronic progressive condition in which the heart cannot generate a cardiac output sufficient to meet the body’s demands without increasing diastolic pressure resulting from any cardiac disease compromising ventricular systolic or diastolic function. The common term for describing heart failure is based on left ventricular ejection fraction.
Also, heart failure with normal LVEF (≥50%) is known as heart failure with preserved ejection fraction and heart failure with decreased LVEF (<40%) as heart failure with reduced ejection fraction. Heart failure with a 40–49% middle range is known as heart failure with mid-range ejection fraction (HFmrEF). The signs and symptoms of Congestive Heart Failure are subtle at the initial stage, and these are generally misguided for common signs of aging. The common symptoms of Congestive Heart Failure are because of extra fluid or congestion, which leads to vessel blocking. The starting of the congestion in the lungs is forwarded to the different body parts.
Congestive Heart Failure Diagnosis
The assessment for Congestive Heart Failure is achieved using numerous parameters, like physical testing of the patient to know the occurrence of clinical symptoms of HF, various blood tests, urine analysis, fasting glucose and lipid profile, metabolic profiling for serum electrolytes, and thyroid hormone estimation. Apart from these routine tests, different imaging techniques like chest X-ray, ECG, MRI, and others are used for diagnosing Congestive Heart Failure.
The patient’s journey begins with the onset of symptoms like breathlessness at night, congestion of the lungs, difficulty in walking, and edema in the legs. Following an initial visit with a general practitioner, during which the patient underwent a complete physical examination, and the results revealed a few alarming findings related to Congestive Heart Failure, the patient was referred to a cardiologist. Further, the cardiologist will immediately recommend a blood examination, assessment of brain natriuretic peptide (BNP) or N-terminal pro-b-type BNP (NT-proBNP), and ECG is prescribed to diagnose heart failure. If the test result shows an increased BNP level and an abnormal ECG—the possibility of heart failure is confirmed. Once heart failure is confirmed an echocardiogram is done to measure the ejection fraction, which helps in identifying causes and stages of heart failure, and also helps in undertaking proper treatment decisions.
Note: Further details related to diagnosis are provided in the report...
Congestive Heart Failure Treatment
Currently, Congestive Heart Failure treatment depends on angiotensin-converting enzyme inhibitors, angiotensin receptor II blockers, beta-blockers, and diuretics. Additionally, other therapies, such as aldosterone antagonists, amiodarone, antiaggregants, anticoagulants, calcium antagonists, diuretics, and nitrates, among others, are used for the treatment of patients affected by heart failure. In addition to this, the treatment of heart failure should be viewed uninterruptedly. In general, early treatment focuses on lifestyle changes (quitting smoking, avoiding alcohol, caffeine, and stress, reducing fluid intake, and reducing the amount of salt in the diet) and optimization of necessary medical therapies. The next step in treatment might include more intense medical therapies or the installation of a device, such as a pacemaker or implantable cardioverter-defibrillator (ICD).
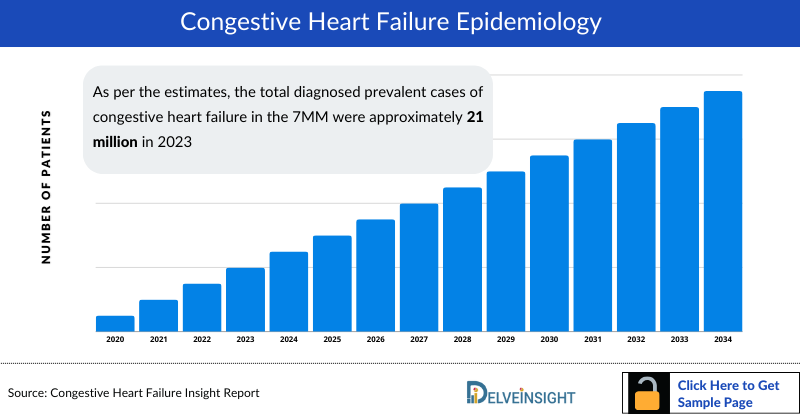
Congestive Heart Failure Epidemiology
As the market is derived using the patient-based model, the Congestive Heart Failure epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of heart failure, gender-specific cases of heart failure, age-specific cases of heart failure, NYHA class-specific cases of heart failure, ejection fraction-specific cases of heart failure, and type-specific cases of heart failure (acute and chronic) in the 7MM covering the United States, EU4 (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key findings
Driven by multiple factors, the number and distribution of reported cases of Congestive Heart Failure have increased over time in the 7MM, among which the United States contributed the major patient share. As per the estimates, the total diagnosed prevalent cases of Congestive Heart Failure in the 7MM were approximately 21 million in 2023, and males are more prevalent than females.
- The analysis of Congestive Heart Failure in the 7MM draws a clear scenario which showed that the patients with HFpEF have a larger proportion (i.e., ~50%) because the heart is not severely affected but keeps on working through medication or symptomatic treatment, whereas, in the case of HFrEF, the patient’s heart is majorly affected. With growing age, the pumping rate of the heart declines.
- In 2023, among all the four New York Heart Association (NYHA) Classes of heart failure, Class II accounted for around 45% of the total diagnosed heart failure cases in the 7MM.
- Maximum cases of heart failure fall in the age group of ≥ 60 years. In 2023, approximately 6.8 million cases of heart failure in the US belonged to this age group.
- In 2023, among EU4 and the UK, the largest number of heart failure cases were observed in Germany, followed by France and Spain, whereas the United Kingdom accounted for the smallest number of cases in the 7MM.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Congestive Heart Failure Prevalence
Recent Developments in the Congestive Heart Failure Treatment Market
- In September 2025, Corstasis Therapeutics Inc. announced that the FDA approved ENBUMYST (bumetanide nasal spray) for the treatment of edema associated with congestive heart failure (CHF), as well as hepatic and renal diseases, including nephrotic syndrome in adults. ENBUMYST offers an enhanced outpatient therapeutic option for patients with cardiovascular and renal conditions.
- In September 2025, Aptar Pharma announced that its Unidose Liquid System has been selected as the delivery platform for Enbumyst™ (Bumetanide Nasal Spray) 0.5mg, recently approved by the U.S. FDA. Developed by Corstasis Therapeutics, Enbumyst™ is the first intranasal loop diuretic approved for the treatment of edema associated with congestive heart failure, liver and kidney diseases, including nephrotic syndrome, in adults.
- In December 2024, Gerresheimer announced that the FDA granted SQ Innovation Tentative Approval for Lasix ONYU for home treatment of fluid overload in congestive heart failure.
Congestive Heart Failure Drug Chapters
The drug chapter segment of the Congestive Heart Failure treatment market report encloses a detailed analysis of Congestive Heart Failure-marketed drugs and late-stage (Phase III and Phase II) Congestive Heart Failure pipeline drugs analysis. It also helps understand the Congestive Heart Failure clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Congestive Heart Failure drugs
- ENTRESTO: Novartis
ENTRESTO combines a neprilysin inhibitor and an angiotensin II receptor blocker. In July 2015, the US FDA approved sacubitril with valsartan to reduce the risk of cardiovascular (CV) death and hospitalization in patients with Congestive Heart Failure (NYHA Class II-IV) associated with reduced ejection fraction and got expanded approval in October 2019 and February 2021 for pediatric patients aged ≥1 with symptomatic heart failure with systemic left ventricular systolic dysfunction and adult patients with Congestive Heart Failure respectively. ENTRESTO captured the major share of the Congestive Heart Failure market in 2022.
- JARDIANCE: Boehringer Ingelheim and Eli Lilly
JARDIANCE is a sodium-glucose cotransporter 2 (SGLT2) inhibitor. The candidate is also being investigated in pivotal trials for heart failure post myocardial infarction and chronic kidney disease. In June 2021, Boehringer Ingelheim and Eli Lilly and Company announced that the European Commission had granted marketing authorization for JARDIANCE (empagliflozin) as a treatment for adults with symptomatic chronic HFrEF. Later in August 2021, it was approved by the US FDA. US FDA had granted Breakthrough Therapy designation based on results from the Phase III EMPEROR-Preserved trial. In the last year, JARDIANCE grabbed regulatory approval from all the major regulatory bodies (FDA, EMA, and PMDA) for the treatment of chronic heart failure in patients with HFpEF, thereby extending its targeted niche.
Note: Detailed Current therapies assessment will be provided in the full report...
Emerging Congestive Heart Failure drugs
KERENDIA (finerenone): Bayer
Finerenone (BAY 94-8862) is an investigational novel, nonsteroidal, selective mineralocorticoid receptor antagonist (MRA) that has been shown to block many harmful effects of mineralocorticoid receptor (MR) overactivation, which is a major driver of kidney and heart damage. The drug has received regulatory approval in the US, Canada, and the EU to reduce the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, nonfatal myocardial infarction, and hospitalization for heart failure in adult patients with chronic kidney disease associated with type 2 diabetes. The drug is currently in a Phase III FINEARTS-HF trial in patients with heart failure and left ventricular ejection fraction greater or equal to 40%. FINEARTS-HF is currently the largest outcome trial investigating an MRA in HFpEF.
For More Information of this Report @ KERENDIA Market
MOUNJARO (tirzepatide): Eli Lilly and Company
Tirzepatide (LY3298176) is a once-weekly dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that integrates the actions of both incretins into a single novel molecule. In May 2022, tirzepatide was approved by the US FDA as a treatment for adults with type 2 diabetes. Currently, the drug is in the Phase III stage of the clinical development program evaluating its safety and efficacy in participants with HFpEF and obesity. This drug has true potential and is expected to garner the maximum revenue by 2034.
Learn More of this Report @ TIRZEPATIDE Market
Congestive Heart Failure Drugs Market Insights
The primeval drug classes like ACE inhibitors, ARBs, beta-blockers, and diuretics are still at the forefront of the heart failure treatment space. Furthermore, off-label usage of other therapies such as aldosterone antagonists, amiodarone, antiaggregants, anticoagulants, calcium antagonists, and nitrates, are also high in patients affected by heart failure.
The majority of therapies are prescribed in combination, and beta-blockers are one of the most prescribed classes. Among the approved therapies, ENTRESTO is Novartis' one of the most important drugs, and its revenue is expected to rise in the next few years. At present, ENTRESTO leads the Congestive Heart Failure market among the approved therapies. After a slow beginning, ENTRESTO has emerged as one of Novartis' most important growth drivers. With the current approval in preserved ejection fraction, ENTRESTO is getting bigger and better.
For many years, ENTRESTO had unparalleled access to the Congestive Heart Failure market; nevertheless, the introduction of SGLT2 inhibitors (FARXIGA, and JARDIANCE), VERQUVO, and other new medicines will pose a threat to this billion-dollar medication. In addition to this, Novartis expects to lose exclusivity in the United States in 2025. There is a threat of generics. Several patent lawsuits are being pursued by Novartis.
SGLT2 inhibitors have also made inroads into the Congestive Heart Failure market. Even though JARDIANCE and FARXIGA are old candidates, they are new to the Congestive Heart Failure market. The future of SGLT-2 drugs in heart failure appears to be promising. Even though both medications appear to be promising, AstraZeneca and Lilly/Boehringer will not have much time to convince heart specialists because FARXIGA's US patent expires in 2025 and JARDIANCE's expires in 2028. Sotagliflozin is a new SGLT2 inhibitor, and gaining market share from older medications such as FARXIGA and JARDIANCE could be difficult.
Congestive Heart Failure Market Outlook
After a scarcity of sustainable new therapies for more than a decade, new classes of agents for the treatment of patients with Congestive Heart Failure were approved by the US FDA – ENTRESTO (sacubitril/valsartan), a combined angiotensin receptor-neprilysin inhibitor (ARNI) and CORLANOR (ivabradine), a sinoatrial node modulator. Both drugs are recommended for use as part of a comprehensive medical therapy regimen. Recently approved VERQUVO (vericiguat), from Merck Pharma, in January 2021 is the newest addition for treating heart failure.
The drug is indicated to reduce the risk of cardiovascular death, heart failure hospitalizations, or the need for outpatient intravenous diuretics. It is recommended in adults with symptomatic chronic heart failure and less than 45% ejection fraction. VERQUVO is a stimulator of soluble guanylate cyclase. In a recent update from the Heart Failure Association on SGLT2 inhibitors in heart failure, dapagliflozin or empagliflozin are recommended to reduce the risk of heart failure hospitalization and cardiovascular death in HFrEF patients already receiving guideline-directed medical therapy, regardless of the presence of type 2 diabetes mellitus.
Therefore, the current Congestive Heart Failure Treatment Market Landscape for this patient pool includes a range of drugs with a variable Congestive Heart Failure mechanism of action, offering safe and tolerable treatment options. Recently, some approved drugs also got the label expansion for treating Congestive Heart Failure in pediatric and adult patients. Many major companies are investing in different mechanisms of action for the treatment of Congestive Heart Failure, SGLT2 inhibitors, cardiac myosin activators, myeloperoxidase inhibitors, mineralocorticoid receptor antagonists, GLP-1 receptor agonists, cell therapies, and others, which will boost the Congestive Heart Failure market in the future.
Conclusively, the emerging pipeline for treating patients with Congestive Heart Failure is filled with potential drugs and cell therapies that can significantly capture a major market share, once launched into the Congestive Heart Failure treatment space. Upcoming drugs, such as finerenone, tirzepatide (LY3298176), semaglutide, omecamtiv mecarbil, and others are expected to shed more light on the importance of novel mechanisms, which have not been approved in the Congestive Heart Failure market so far.
Key Congestive Heart Failure Companies such as Bayer (finerenone), Eli Lilly and Company (tirzepatide or LY3298176), Novo Nordisk A/S (semaglutide), BioCardia (CardiAMP Cell Therapy), Mesoblast (rexlemestrocel-L), scPharmaceuticals (FUROSCIX Infusor), Cytokinetics (omecamtiv mecarbil), Lexicon Pharmaceuticals (sotagliflozin) and others are evaluating their lead candidates in different stages of clinical development, respectively.
- The total Congestive Heart Failure Treatment Market Size in the 7MM was approximately USD 6,900 million in 2023 and is projected to grow during the forecast period (2024-2034).
- Among the 7MM the United States contributed the largest Congestive Heart Failure Treatment Market Size and will continue to experience a steep rise in the market size throughout the forecast period.
- In 2023, among the EU4 and the UK, Germany has the maximum revenue share, while the United Kingdom has the smallest market share.
- In the 7MM, ENTRESTO is expected to garner the largest market share among the marketed therapies during the forecast period, followed by beta blockers. Among the emerging drugs semaglutide and tirzepatide are expected to grab a decent market share compared to others in the pipeline.
- The use of stem cells to stimulate myocardial healing is regarded as a new approach to treating heart failure. The late-stage cell therapy CardiAMP Cell Therapy secured the third position in generating maximum revenue during the forecast period, in Japan.
Congestive Heart Failure Drug Uptake
This section focuses on the uptake rate of the potential Congestive Heart Failure drugs recently launched in the Congestive Heart Failure market or expected to be launched during the study period 2020–2034. For example, for tirzepatide, we expect the drug uptake in the US to be medium-fast with a probability-adjusted peak share of 1.4%, years to the peak is expected to be 8 years from the year of launch.
The analysis covers the Congestive Heart Failure therapeutics market uptake by drugs, patient uptake by therapies, and sales of each drug. This will help in understanding the Congestive Heart Failure drugs with the most rapid uptake and the reasons behind the maximal use of new drugs and allows the comparison of the drugs based on market share and size, which again will be useful in investigating factors important in the market uptake and in making financial and regulatory decisions.
Congestive Heart Failure Pipeline Development Activities
The Congestive Heart Failure therapeutics market report provides insights into Congestive Heart Failure clinical trials within Phase III, Phase II, and Phase I/II stage. It also analyzes key Congestive Heart Failure Companies involved in developing targeted therapeutics. The report covers information on collaborations, acquisitions, and mergers, licensing patent details, and other information on emerging Congestive Heart Failure therapies.
KOL Views
To keep up with current epidemiology and market trends, we take KOLs and SMEs’ opinions working in the Congestive Heart Failure domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Congestive Heart Failure's evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, include Medical/scientific writers, Cardiologists, and Professors: MD, Cardiologist at Mayo Clinic, Cardiologist and HOD of Mount Sinai Hospital, and Others.
Delveinsight’s analyst connected with 50+ KOLs (cardiologists, physicians, researchers, principal investigators, etc.) across the 7MM. Centers such as Mount Sinai Hospital, Mayo Clinic, Southern Illinois University; University of Verona, Verona, Italy; University of Valencia and INCLIVA Biomedical Research Institute, Valencia, Spain; and others were contacted. Their opinion helps to understand and validate current and emerging therapies, treatment patterns, and Congestive Heart Failure market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
KOL Views
United States “Previous studies have identified some characteristics of these lipid deposits which put them at high risk of rupturing. These studies involved examining the obstructed coronary arteries of patients who died from infarction or using special imaging techniques to view these vessels from the inside. But the exact mechanism of plaque rupture, and especially the forces at work behind it, are still not known.”
United Kingdom “Standardized but limited point-of-care echocardiograms are helpful in early triage and identification of patients presenting with cardiovascular symptomatology such as abnormal electrocardiograms that at times mimicked acute myocardial infarctions or in patients with elevated cardiac biomarkers.”
Congestive Heart Failure Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided. Reducing the risk of the combined endpoint of cardiovascular death or hospitalization for heart failure, based on a time-to-event analysis, played an important role as a part of the efficacy parameter. Moreover, the adverse events during the analysis are majorly observed as safety parameters. The ranking of drugs and cell therapies was done accordingly.
Congestive Heart Failure Therapeutics Market Access and Reimbursement
The burden of heart failure is multifaceted, including impacts on quality of life, medical comorbidities, and financial costs to patients and the health care system. Medications constitute the majority of direct costs for patients with heart failure. Many nonprofit organizations, companies, government hospitals, and private healthcare insurance companies provide low-cost copay programs to reduce the financial burden of medication on the patient. By receiving financial assistance, patients can focus on improving their health and overall quality of life.
According to the NICE UK, ENTRESTO is recommended as an option for treating symptomatic chronic heart failure with reduced ejection fraction only in people:
- With New York Heart Association (NYHA) Class II-IV symptoms
- With a left ventricular ejection fraction of 35% or less
- Who are already taking a stable dose of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor-blockers (ARBs)
This guidance is not intended to affect the position of patients whose treatment with sacubitril valsartan was started within the NHS before this guidance was published. Treatment of those patients may continue without change to whatever funding arrangements were in place for them before this guidance was published until they and their NHS clinician consider it appropriate to stop (NICE, 2016).
The Congestive Heart Failure Therapeutics Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Congestive Heart Failure Therapeutics Market Report Scope
- The Congestive Heart Failure therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current Congestive Heart Failure Treatment Market Landscape.
- A detailed review of the Congestive Heart Failure therapeutics market, historical and forecasted Congestive Heart Failure treatment market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Congestive Heart Failure therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Congestive Heart Failure drugs market.
Congestive Heart Failure Therapeutics Market Report Insights
- Patient-based Congestive Heart Failure Market Forecasting
- Congestive heart failure Therapeutic Approaches
- Congestive Heart Failure Pipeline Drugs Analysis
- Congestive Heart Failure Market Size and Trends
- Existing and Future Congestive Heart Failure Drugs Market Opportunity
Congestive Heart Failure Therapeutics Market Report Key Strengths
- 11 years Congestive Heart Failure Market Forecast
- The 7MM Coverage
- Congestive Heart Failure epidemiology segmentation
- Key Cross Competition
- Conjoint Analysis
- Congestive heart failure Drugs Uptake
- Key Congestive heart failure Market Forecast Assumptions
Congestive Heart Failure Therapeutics Market Report Assessment
- Current Congestive Heart Failure Treatment Market practices
- Congestive heart failure Unmet needs
- Congestive heart failure Pipeline Drugs Analysis Profiles
- Congestive heart failure Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Congestive heart failure Market Drivers
- Congestive heart failure Market Barriers
Key Questions Answered In The Congestive Heart Failure Market Report
Congestive Heart Failure Therapeutics Market Insights
- What was the Congestive Heart Failure drugs market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors for this growth?
- What are the key findings of the Congestive Heart Failure drugs market across the 7MM, and which class will account for the largest market share during the forecast period (2024–2034)
- How will the launch of FUROSCIX affect the treatment paradigm in the management of Congestive Heart Failure?
- Will the label expansion of ENTRESTO bring a significant impact on its market growth during the forecast period?
- What would the impact of SGLT2 inhibitors be on the treatment paradigm in the management of Congestive Heart Failure?
- What is the impact of off-label usage of conventional therapies on the current Congestive Heart Failure market and what are the prospects? What are the various strategies adopted by the pharma giants to tackle this threat?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Congestive Heart Failure Epidemiology Insights
- What are the disease risks, burdens, and Congestive Heart Failure Unmet Needs? What will be the growth opportunities across the 7MM concerning the patient population of Congestive Heart Failure?
- What is the historical and forecasted Congestive Heart Failure patient pool in the 7MM, and where can one observe the highest patient population and growth opportunities?
- What are the key factors driving the epidemiology trends for seven major markets covering the United States, EU4 (Germany, Spain, France, Italy), the UK, and Japan?
- Which age group accounts for the major share of the diagnosed Congestive Heart Failure population?
- Which Class of Congestive Heart Failure is the largest contributor to the total Congestive Heart Failure patient pool among all the four NYHA Classes?
- What factors are contributing to the rise in the prevalence rate of Heart failure?
Current Congestive Heart Failure Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Congestive Heart Failure? What are the current guidelines for treating Congestive Heart Failure in the US and Europe?
- How many companies are developing therapies for the treatment of Congestive Heart Failure?
- How many emerging therapies are in the mid-stage and late stage of development for treating Congestive Heart Failure?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for Congestive Heart Failure Emerging Therapies?
- What is the cost burden of approved therapies on the patient?
- What are the key designations that have been granted for Congestive Heart Failure Emerging Therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What is the 7MM historical and forecasted Congestive Heart Failure Drugs Market?
Reasons to Buy
- The Congestive Heart Failure therapeutics market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Congestive Heart Failure drugs market.
- Insights on patient burden/disease Congestive Heart Failure prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Congestive Heart Failure drugs market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Congestive Heart Failure drugs market will help devise strategies that will help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Congestive Heart Failure drugs market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles:-
-market.png&w=256&q=75)

.png&w=3840&q=75)
