Corneal Endothelial Dystrophy Market
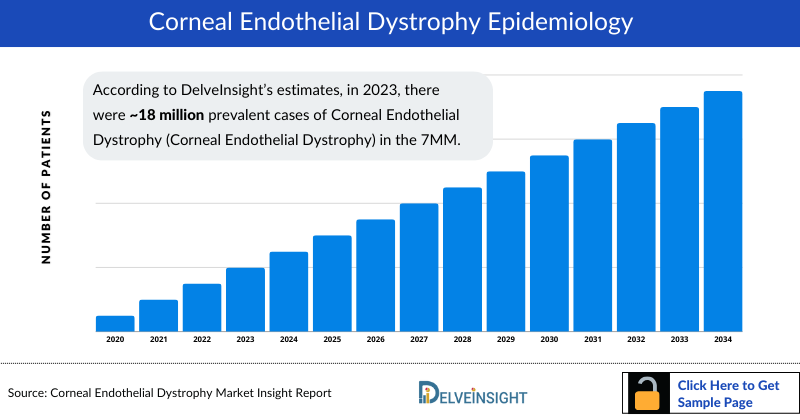
- According to DelveInsight’s estimates, in 2023, there were approximately 18 million prevalent cases of Corneal Endothelial Dystrophy (Corneal Endothelial Dystrophy) in the 7MM. Of these, the US accounted for approximately 37% of the cases, EU4 and the UK countries accounted for around 46%, followed by Japan which represented nearly 16%.
- The Corneal Endothelial Dystrophy market is set for steady growth, with a robust compound annual growth rate (CAGR) of 8% anticipated from 2024 to 2034. This expansion in the 7MM is driven by the introduction of innovative therapies such as Sirolimus, and TTHX1114 as well as the aging population, growing awareness and diagnosis, and innovation in pharmaceutical treatments.
- A significant unmet need in Corneal Endothelial Dystrophy lies in the limited availability of effective, non-invasive FDA-approved drugs. Current options primarily involve corneal transplants, which carry risks and challenges, highlighting the necessity for therapeutic advancements targeting endothelial regeneration and long-term disease management.
- A significant market barrier for Corneal Endothelial Dystrophy is the high cost and limited accessibility of corneal transplant proCorneal Endothelial Dystrophyures. Additionally, the shortage of donor corneas and specialized surgical expertise further restricts treatment options, creating challenges for the widespread adoption of advanCorneal Endothelial Dystrophy therapeutic solutions.
DelveInsight’s “Corneal Endothelial Dystrophy Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Corneal Endothelial Dystrophy, historical and forecasted epidemiology, as well as the Corneal Endothelial Dystrophy market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Corneal Endothelial Dystrophy market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Corneal Endothelial Dystrophy market size from 2020 to 2034. The report also covers Corneal Endothelial Dystrophy treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
CED Epidemiology |
|
|
CED Market |
|
|
Market Analysis |
|
|
CED Market players |
|
|
Future opportunity |
The future opportunity in addressing CED lies in the development of innovative pharmacological therapies aimed at enhancing corneal endothelial cell regeneration and function. Advances in gene therapy and stem cell technology could pave the way for non-surgical interventions, potentially reducing reliance on corneal transplants and improving patient outcomes. Furthermore, the increasing understanding of the molecular mechanisms underlying CED offers a promising pathway for targeted treatments, positioning stakeholders to capitalize on emerging therapeutic modalities that address both the symptoms and root causes of this condition. |
Corneal Endothelial Dystrophy Treatment Market
Corneal Endothelial Dystrophy overview
Corneal Endothelial Dystrophy, specifically Fuchs’ Endothelial Dystrophy (FED), is a rare, genetically-driven disorder affecting the endothelial layer of the cornea. It manifests through the accumulation of foreign material, primarily in the form of guttae deposits, which contribute to cell death within the cornea. This leads to corneal edema, resulting in visual disturbances such as glare, halos, and reduCorneal Endothelial Dystrophy acuity. FED is progressive, bilateral, and predominantly affects females. The disease's advanCorneal Endothelial Dystrophy stages may involve the development of corneal blisters, which, upon bursting, cause significant pain and further visual deterioration, potentially leading to blindness.
Corneal Endothelial Dystrophy diagnosis
Diagnosis of Corneal Endothelial Dystrophy, particularly FED, involves a comprehensive clinical examination, primarily using slit-lamp biomicroscopy to detect characteristic guttae in the corneal endothelium. Specular microscopy or confocal microscopy is employed to assess endothelial cell density and morphology. Corneal pachymetry measures corneal thickness to evaluate edema progression, while optical coherence tomography (OCT) provides cross-sectional imaging for detailed corneal layer analysis. Additionally, visual acuity testing and corneal topography may be performed to assess visual function and structural changes. These diagnostic tools facilitate early detection and staging of the disease.
Further details related to country-based variations are provided in the report…
Corneal Endothelial Dystrophy treatment
Corneal Endothelial Dystrophy is primarily managed through interventions aimed at improving corneal clarity and preserving vision. Pharmacological options, such as hypertonic saline drops, reduce corneal edema by drawing out excess fluid, offering temporary relief. However, for advanCorneal Endothelial Dystrophy cases, surgical solutions like Descemet's Stripping Automated Endothelial Keratoplasty (DSAEK) or Descemet Membrane Endothelial Keratoplasty (DMEK) remain definitive treatments, replacing damaged endothelial cells. Non-pharmacological measures, including scleral lenses, are employed to enhance visual acuity by reducing corneal distortion. These approaches provide a spectrum of care based on disease severity, with surgery being the most effective for long-term vision restoration.
Corneal Endothelial Dystrophy Epidemiology
As the market is derived using a patient-based model, the Corneal Endothelial Dystrophy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total prevalent cases of Corneal Endothelial Dystrophy, gender-specific cases of Corneal Endothelial Dystrophy, age-specific cases of Corneal Endothelial Dystrophy and severity-specific cases of Corneal Endothelial Dystrophy in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- According to DelveInsight’s epidemiology model, in the US, the total prevalent cases of Corneal Endothelial Dystrophy were approximately 6.6 million in 2023. This number is anticipated to rise during the forecast period (2024-2034), driven by the aging population, genetic predisposition, and improved diagnostic techniques.
- Among the EU4 and the UK, Germany accounted for the highest number of prevalent cases of Corneal Endothelial Dystrophy, with approximately 2.1 million cases in 2023, followed by Italy and France with approximately 1.6 million cases.
- Among the gender-specific cases of Corneal Endothelial Dystrophy in the US in 2023, there were approximately 4.7 million cases for females and around 1.9 million cases for males.
- In 2023, the age-specific cases in Japan were categorized into 40–49 years, 50–59 years, 60–69 years, 70–79 years, and >80 years, from which the 50–59 years age group had the highest number of prevalent cases of Corneal Endothelial Dystrophy among the 7MM, totaling approximately 869 thousand cases.
- Among the severity-specific prevalent cases of Corneal Endothelial Dystrophy in Japan in 2023, there were approximately 2.7 million mild cases and around 144 thousand moderate to severe cases.
Corneal Endothelial Dystrophy Drug Chapters
Corneal Endothelial Dystrophy Emerging Drugs
Sirolimus: Santen Pharmaceutical
In November 2021, Santen Pharmaceutical and ActualEyes entered a joint development agreement to advance Phase II clinical trials for sirolimus eye drops (STN1010904/AE-001) targeting FCorneal Endothelial Dystrophy. The trial, involving approximately 80 patients aged 30 to 75, is a multi-center, double-blind, randomized, placebo-controlled study conducted across the US and France. The study evaluates two concentrations of STN1010904/AE-001, focusing on best-corrected visual acuity, low-contrast visual acuity, and contrast sensitivity to assess the agent's efficacy and safety in treating FECD.
TTHX1114: Trefoil Therapeutics
TTHX1114, an investigational engineered variant of Fibroblast Growth Factor-1 (FGF1), promotes cell proliferation, migration, and protection against stress and injury. Its topical formulation aims to expedite corneal ulcer healing by stimulating epithelial cell growth, potentially minimizing complications like pain, inflammation, and corneal scarring. Trefoil Therapeutics is also developing an intracameral injection of TTHX1114 for treating FCorneal Endothelial Dystrophy and other corneal endothelial dystrophies, focusing on regenerating lost endothelial cells to improve vision. In April 2023, Trefoil presented data at ARVO demonstrating TTHX1114’s ability to control edema in cataract surgery patients at risk. Trefoil holds licensed patents from Florida State University and has expanded its intellectual property with company-developed technology.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Sirolimus |
mTOR inhibitor |
Topical |
Santen Pharmaceutical |
IIa |
|
TTHX1114 |
FGF1 derivative |
Intracameral injection |
Trefoil Therapeutics |
II |
|
XX |
XX |
XX |
XXX |
X |
Note: Further emerging therapies and their detailed assessment will be provided in the final report...
Drug Class Insights
Drugs involved in the treatment of Corneal Endothelial Dystrophy primarily belong to the class of hyperosmotic agents, such as hypertonic saline drops. These agents work by creating an osmotic gradient that draws fluid out of the cornea, temporarily reducing corneal edema and improving vision clarity. While effective in managing early symptoms, their relief is typically transient, addressing fluid buildup without altering disease progression. In cases of secondary inflammation or irritation, lubricants or anti-inflammatory agents may be used adjunctively to protect the corneal surface and manage discomfort, though these do not target the underlying endothelial dysfunction.
Continued in report...
Corneal Endothelial Dystrophy Market Outlook
The treatment landscape for Corneal Endothelial Dystrophy (Corneal Endothelial Dystrophy) remains focused on symptomatic management and surgical interventions, with corneal transplantation serving as a critical option for advanCorneal Endothelial Dystrophy cases. Current therapies, such as topical hypertonic saline drops, provide temporary relief from corneal edema but do not alter disease progression. Surgical advancements, particularly in endothelial keratoplasty techniques like DSAEK and DMEK, have significantly improved patient outcomes by reducing complications and allowing for earlier intervention. Emerging therapies, such as sirolimus eye drops, are being explored for their potential to address the underlying pathophysiology of Fuchs Endothelial Corneal Dystrophy by promoting endothelial cell health. Additionally, TTHX1114, an engineered variant of FGF1, is under investigation for its ability to accelerate corneal wound healing and protect endothelial cells. These innovative approaches could enhance the current treatment paradigm, offering hope for improved management of Corneal Endothelial Dystrophy and the potential to change the disease trajectory.
Continued in report...
Corneal Endothelial Dystrophy Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. For example, TTHX1114 is expected to enter the US market in 20XX and is projected to have a XX uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report...
Corneal Endothelial Dystrophy Pipeline Development Activities
The report provides insights into different Corneal Endothelial Dystrophy clinical trials within Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Corneal Endothelial Dystrophy.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Corneal Endothelial Dystrophy's evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Jules Stein Eye Institute/UCLA; UCL Institute of Ophthalmology; Department of Pharmacology and Biochemistry, UT Southwestern Medical Center at Dallas, Dallas, Texas; Department of Ophthalmology, University Hospital of Cologne, Cologne, Germany among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Corneal Endothelial Dystrophy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per the KOLs from Germany, Therapeutic options are currently limited, as is the case for many neurodegenerative diseases, but new therapeutic approaches including gene therapy, stem cell therapy, Optogenetics, and retinal prostheses are on the horizon. Many of these approaches have entered the clinical phase of development.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further details will be provided in the report...
Scope of the Corneal Endothelial Dystrophy Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Corneal Endothelial Dystrophy explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Corneal Endothelial Dystrophy market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Corneal Endothelial Dystrophy market.
Corneal Endothelial Dystrophy report insights
- Corneal Endothelial Dystrophy Patient Population
- Corneal Endothelial Dystrophy Therapeutic Approaches
- Corneal Endothelial Dystrophy Pipeline Analysis
- Corneal Endothelial Dystrophy Market Size and Trends
- Existing and Future Market Opportunity
Corneal Endothelial Dystrophy report key strengths
- 11 years Forecast
- The 7MM Coverage
- Corneal Endothelial Dystrophy Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Corneal Endothelial Dystrophy Drugs Uptake
- Key Corneal Endothelial Dystrophy Market Forecast Assumptions
Corneal Endothelial Dystrophy report assessment
- Current Corneal Endothelial Dystrophy Treatment Practices
- Corneal Endothelial Dystrophy Unmet Needs
- Corneal Endothelial Dystrophy Pipeline Product Profiles
- Corneal Endothelial Dystrophy Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Corneal Endothelial Dystrophy Market Drivers
- Corneal Endothelial Dystrophy Market Barriers
Key Questions
Corneal Endothelial Dystrophy Market Insights
- What was the total market size of Corneal Endothelial Dystrophy, the market size of Corneal Endothelial Dystrophy by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will TTHX1114 affect the treatment paradigm of Corneal Endothelial Dystrophy?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Corneal Endothelial Dystrophy Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Corneal Endothelial Dystrophy? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Corneal Endothelial Dystrophy?
- What is the historical and forecasted Corneal Endothelial Dystrophy patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Corneal Endothelial Dystrophy population during the forecast period (2024–2034)?
- What factors are contributing to the growth of Corneal Endothelial Dystrophy cases?
Current Corneal Endothelial Dystrophy Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Corneal Endothelial Dystrophy? What are the current clinical and treatment guidelines for treating Corneal Endothelial Dystrophy?
- How many companies are developing therapies for the treatment of Corneal Endothelial Dystrophy?
- How many emerging therapies are in the mid-stage and late stage of development for treating Corneal Endothelial Dystrophy?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of Corneal Endothelial Dystrophy?
Reasons to Buy Corneal Endothelial Dystrophy Market Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Corneal Endothelial Dystrophy market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Corneal Endothelial Dystrophy, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.