Dup15q Syndrome Market
Key Highlights:
- Dup15q syndrome is a neurogenetic condition caused by duplications on chromosome 15q11.2-13.1. It is marked by hypotonia, developmental delays, intellectual disability (ID), epilepsy, and autism spectrum disorder (ASD). The extent of developmental impairments can vary significantly based on the nature of the duplication and whether it originated from the mother or father.
- Dup15q Syndrome is further categorized into two different types: isodicentric chromosome 15 (idic [15]), accounting for about 80% of cases, and interstitial 15q duplication (int dup[15]) occurs in about 20% of cases.
- The prevalence of the disorder in the general population of the US is estimated to be about 1 in 14,000 people, and Dup15q syndrome is one of the most common chromosomal abnormalities in people with ASD, affecting around 1 in 500 people with autism.
- The total prevalent cases of Dup15q syndrome in Japan was found to be approximately 8,900 in 2024.
- Pipeline activity for Dup15q syndrome is not robust. Only a few drugs are in clinical development, such as bexacaserin (Lundbeck) for Developmental and Epileptic Encephalopathies (DEEs). With the recent removal of basmisanil (RG1662) from Roche's pipeline for the treatment of Dup15q syndrome, and the lack of therapies in the pipeline, the market growth of Dup15q syndrome could be hampered.
- Takeda is developing soticlestat for Dup 15q epilepsy in Phase II. The trial is completed in 2020 and company has not provided any information for further developmet. But in January 2025, Takeda decide to discontinue its soticlestat (TAK-935) due to trial failure in Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS).
- Companies like Quiver Bioscience are in preclinical stages of development. Apart from this, Acadia Pharmaceuticals/Saniona's SAN2465, a novel rapid-acting antidepressant candidate, has been selected for preclinical development in major depressive disorders (MDD).
- The Acadia/Saniona deal secures financing enabling Saniona to start Phase II for SAN2465 in MDD. Please note that, as per the company, this drug has an additional opportunity to treat the neuropsychiatric symptoms in Dup15q syndrome (although at the moment no trial is ongoing).
- Dup15q is a complex condition, but with continued scientific progress, innovative partnerships, strong community backing, and unwavering dedication, the prospect of developing disease-modifying treatments is steadily becoming more attainable.
DelveInsight's ‘Dup15q Syndrome – Market Insights, Epidemiology and Market Forecast – 2034’ report delivers an in-depth understanding of the Dup15q Syndrome, historical and forecasted epidemiology as well as the Dup15q Syndrome market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The Dup15q Syndrome market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Dup15q Syndrome market size from 2020 to 2034. The report also covers current Dup15q Syndrome treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Dup15q Syndrome |
Segmented by ● Total Prevalent Cases of Dup15q Syndrome in the 7MM [2020–2034] ● Total Diagnosed Prevalent Cases of Dup15q Syndrome in the 7MM [2020–2034] ● Type-specific Diagnosed Prevalent Cases of Dup15q Syndrome in the 7MM [2020–2034] |
|
Dup15q Syndrome Companies |
● Lundbeck ● Quiver Bioscience |
|
Dup15q Syndrome Therapies |
● Bexicaserin ● UBE3A (ASO) |
|
Dup15q Syndrome Market |
Segmented by ● Region ● Therapies |
|
Analysis |
● KOL views ● SWOT analysis ● Reimbursement ● Conjoint analysis ● Unmet need |
Dup15q Syndrome Understanding and Treatment Algorithm
Dup15q Syndrome Overview
Chromosome 15q11.2-13.1 duplication syndrome (Dup15q syndrome) is a rare genetic disorder that results from duplications of a portion of the 15 chromosome. The portion involves a small region within the long arm (q), 15q11.2-13.1. These duplications most commonly occur in one of two forms. These include an extra idic(15), or an int dup(15). This region is also known as “Prader-Willi/Angelman critical region (PWACR) because it is involved in these syndromes, Prader-Willi and Angelman syndrome.
Dup15q syndrome is characterized by low muscle tone (hypotonia) and gross and fine motor delays, variable intellectual disability (ID), autism spectrum disorder (ASD), and epilepsy, including infantile spasms. These signs and symptoms may differ significantly between affected people, and it is influenced by whether the duplication is inherited from an individual’s mother or father (parent-of-origin) and by the number of copies of the PWACR.
Dup15q Syndrome Diagnosis
Dup15q syndrome is diagnosed through genetic testing that identifies duplications on chromosome 15q11.2-13.1. Clinical evaluation includes assessing developmental milestones, intellectual disability, and autism spectrum disorder symptoms. Genetic counseling may also be provided to understand the implications of the duplication and guide management strategies.
The different genomic testing methods available to determine the copy number of sequences and identify chromosomal duplications can include chromosomal microarray analysis (CMA) and targeted duplication analysis, as well as fluorescent in situ hybridization (FISH) analysis, quantitative PCR (qPCR), and multiplex ligation-dependent probe amplification (MLPA). In addition, the 15q11.1-13.1 region encompasses imprinted genes, depending on the parent-of-origin. This imprinting allows for diagnosis based on methylation status using methylation-sensitive (MS) multiplex ligation-dependent probe amplification (MLPA). MS-MLPA can also efficiently distinguish the parental origin of the duplications of 15q11.2-q13.1.
Further details related to country-based variations are provided in the report.
Dup15q Syndrome Treatment
There is no specific treatment for Dup15q syndrome. This includes antiepileptic medications, physical, occupational, and speech therapies, and applied behavioral analysis (ABA) to address developmental delays, epilepsy, and autism spectrum disorder. Affected people should be seen by several specialists who should evaluate infants for motor and speech development and provide appropriate referrals to educational programs later. Supportive care may include:
- Occupational and physical therapy to improve motor skills
- Alternative and augmentative communication methods
- Behavioral therapy, such as ABA
- Psychotropic medications to manage behavioral issues
- Seizure management using standard treatments
Ongoing monitoring is recommended, including:
- Neurodevelopmental assessments to track progress
- Regular monitoring for signs of seizures or changes in seizure types
Emotional and behavioral therapies address behavioral challenges, such as frustration from communication difficulties, compulsive behaviors, and ADHD, by understanding triggers and developing strategies to encourage positive responses and prevent undesirable behaviors. These limited options are currently available to manage Dup15q syndrome symptoms.
Genetic testing for siblings of a person with Dup15q syndrome (who may be at risk for inheriting a maternal interstitial duplication) is recommended. Early diagnosis allows for timely multidisciplinary evaluation and support.
Further details related to country-based variations are provided in the report.
Dup15q Syndrome Epidemiology
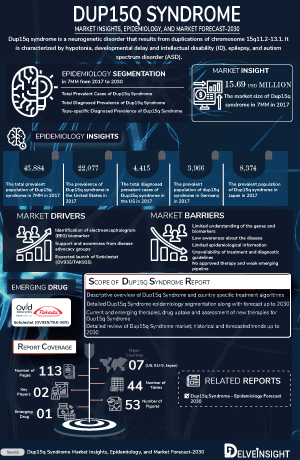
As the market is derived using a patient-based model, the Dup15q Syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total prevalent cases of Dup15q syndrome in the 7MM, total diagnosed prevalent cases of Dup15q syndrome in the 7MM, and type-specific diagnosed prevalent cases of Dup15q syndrome in the 7mm covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034. The total prevalent cases of Dup15q syndrome in the 7MM comprised approximately 54,000 cases in 2024 and are projected to increase during the forecast period.
- In 2024, the US reported the highest prevalent cases of Dup15q Syndrome among the 7MM, with approximately 24,000 cases; these numbers are expected to rise gradually throughout the forecast period (2025–2034).
- According to DelveInsight's analysis, Japan had around 9,000 prevalent cases of Dup15q Syndrome in 2024..
- Among type specific cases, idic (15) had the highest prevalent cases than int dup(15) duplication.
Dup15q Syndrome Drug Chapters
The drug chapter segment of the Dup15q syndrome report encloses a detailed analysis of Dup15q syndrome marketed drugs and late-stage (Phase III, Phase II/III, Phase II, Phase I/II, and Phase I) pipeline drugs. It also helps understand the Dup15q syndrome clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Emerging Drugs
Bexicaserin: Lundbeck
Bexicaserin, previously known as LP352, is an oral therapy designed to bind to 5-HT2C, a receptor in the brain and spinal cord that primarily binds serotonin, a chemical messenger that helps nerve cells communicate. This activation is expected to ease the abnormal electrical activity in the brain that fuels seizures. It is currently being evaluated in a global Phase III program called DEEp. The company is expecting to launch bexicaserin in the fourth quarter of 2028.
In February 2025, the company announced the 12-month data from the Phase Ib/IIa PACIFIC trial showed that treatment with investigational bexicaserin resulted in significant reductions of 57.7% in countable motor seizures in patients with DEEs.
In December 2024, Lundbeck announced the successful completion of the previously announced transaction to acquire all of the outstanding shares of Longboard Pharmaceuticals. Through the acquisition of Longboard, Lundbeck gains access to bexicaserin, a novel 5-HT2C agonist in development for the treatment of seizures associated with DEEs.
In July 2024, the US Food and Drug Administration (FDA) granted Breakthrough Therapy designation for its investigational drug bexicaserin for the treatment of seizures associated with DEEs for patients two years of age or older.
UBE3A (ASO): Quiver Bioscience
Maternal Dup15q syndrome is characterized by hypotonia and motor delays, variable intellectual disability, autism spectrum disorder, and epilepsy. Although ∼40 genes are located in the PWACR, evidence supports the overexpression of the ubiquitin-protein E3A ligase (UBE3A) gene as the predominant molecular cause of the phenotypes observed in Dup15q syndrome. Therefore, the lowering of UBE3A expression by antisense oligonucleotides (ASOs) might be able to reduce the severity of the symptoms. This therapy is in the preclinical development phase.
The pipeline for Dup15q syndrome is very sparse and limited, potentially indicating the limited interest of market players to invest in this disease and simultaneously providing an opportunity to the dominant player to introduce the first approved therapy for Dup15q syndrome and capture a large market.
|
Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
Bexicaserin |
Lundbeck |
III |
Treatment of Seizures in Children and Adults With Developmental and Epileptic Encephalopathies |
Oral |
5-HT2C serotonin receptor agonists |
Small molecule |
Note: Detailed emerging therapies assessment will be provided in the final report.
Drug Class Insights
5-HT2C serotonin receptor agonists
5-HT2C serotonin receptor agonists, such as bexacaserin, play a crucial role in the treatment of Dup15q syndrome given their role in regulating neural excitability. These compounds act by activating 5-HT2C receptors, which can help reduce excessive neuronal firing and restore the excitatory-inhibitory balance in the brain. This is particularly relevant in Dup15q, where abnormal neural signaling contributes to symptoms such as seizures and behavioral disturbances. Preclinical research suggests that targeting this receptor may lead to improvements in neurological function, offering a promising therapeutic avenue. Further clinical studies are needed to fully evaluate their efficacy and safety in individuals with Dup15q.
Dup15q Syndrome Market Outlook
Supportive care for Dup15q syndrome encompasses a range of interventions tailored to the individual's needs. Occupational and physical therapies address motor delays, while alternative and augmentative communication aids support language development. Behavioral therapies, such as applied behavioral analysis (ABA), help manage autism-related symptoms. Psychotropic medications may be used for behavioral challenges, and seizure management includes standard anticonvulsant medications, vagus nerve stimulation, or ketogenic diets to reduce seizure frequency and severity. The therapeutic pipeline for Dup15q syndrome remains limited, with only a few drugs and therapies currently in development. Bexacaserin (Lundbeck), while targeting DEEs, is addressing a broader market rather than focusing specifically on Dup15q. Therapies such as SAN2465, developed by Saniona, originally developed for major depressive disorder, have demonstrated potential to address neuropsychiatric symptoms associated with Dup15q. Additionally, UBE3A (ASO) by Quiver Bioscience is in preclinical development; it utilizes antisense oligonucleotides (ASOs) to reduce UBE3A expression, which may contribute to alleviating the severity of symptoms linked to the syndrome.
- The US accounts for the largest market size of Dup15q, in comparison to EU4 and the UK (Germany, France, Italy, the UK, and Spain) and Japan.
- In February 2025, Lundbeck announced the 12-month data from the Phase Ib/IIa PACIFIC trial showed that treatment with investigational bexicaserin resulted in significant reductions of 57.7% in countable motor seizures in patients with DEEs.
Further details will be provided in the report….
Dup15q Syndrome Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. The analysis covers Dup15q syndrome market uptake by drugs, patient uptake by therapies, and sales of each drug.
Further detailed analysis of emerging therapies' drug uptake in the report…
Dup15q Syndrome Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II/III, Phase II, Phase I/II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Dup15q syndrome emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SME's opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the Dup15q syndrome evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including MD, PhD, Instructor, Postdoctoral Researcher, Professor, Researcher, and Others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 6+ KOLs in the 7MM. Centers such as the National Organization for Rare Disorders (NORD), Genetic and Rare Diseases Information Center, Istituto di Ricerche Farmacologiche Mario Negri (IRCCS), etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Dup15q syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
Region |
KOL Views |
|
United States |
“Dup15q syndrome is diagnosed by identifying an additional copy of the Prader-Willi/Angelman critical region (PWACR) on chromosome 15 through various genomic testing methods. Chromosomal microarray analysis measures copy numbers of specific chromosome regions, targeted duplication analysis assesses different DNA segment sizes, and fluorescence in situ hybridization (FISH) distinguishes between extra chromosomes and interstitial duplications." |
|
Italy |
”Dup15q syndrome is a rare genetic condition characterized by a broad range of clinical presentations and varying severity. Despite its clinical heterogeneity, there has been no formal effort to identify specific clusters of symptoms, signs, and diagnostic tests for differentiating it from other neurodevelopmental disorders.” |
Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, a descriptive overview of Dup15q syndrome, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Dup15q syndrome market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Dup15q syndrome market.
Dup15q Syndrome Report Insights
- Patient population
- Therapeutic approaches
- Dup15q syndrome pipeline analysis
- Dup15q syndrome market size and trends
- Existing and future market opportunity
Dup15q Syndrome Report Key Strengths
- Ten-year forecast
- 7MM coverage
- Dup15q syndrome epidemiology segmentation
- Key cross competition
- Highly analyzed market
- Drug uptake
Dup15q Syndrome Report Assessment
- Current treatment practices
- Unmet needs
- Pipeline product profiles
- Market attractiveness
- Qualitative analysis (SWOT and conjoint analysis)
FAQs
Market Insights
- What was the Dup15q syndrome market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of Dup15q syndrome?
- What are the disease risks, burdens, and unmet needs of Dup15q syndrome? What will be the growth opportunities across the 7MM concerning the patient population with Dup15q syndrome?
- What are the current options for the treatment of Dup15q syndrome? What are the current guidelines for treating Dup15q syndrome in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Dup15q syndrome market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand KOLs’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.