EGFR Inhibitors-Induced Skin Disorders Market
- Among the 7MM, the highest market size was occupied by the United States in 2023.
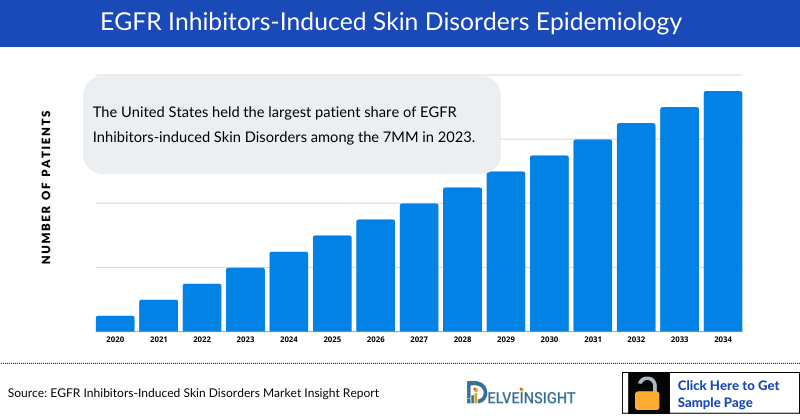
- The United States held the largest patient share of EGFR Inhibitors-induced Skin Disorders among the 7MM in 2023.
- Among EU4 and the UK, Germany had the highest number of total cases of EGFR inhibitors-induced skin disorders followed by France in 2023.
- In February 2024, the US FDA granted Orphan Drug Designation to LUT014 for the treatment of EGFR inhibitors-induced acneiform rash.
- Currently, EGFR inhibitor-induced skin diseases are only diagnosed based on clinical, histologic, and physical examination findings. As the chances of occurrence of skin toxicities are very high with EGFR inhibitors, dermatologists should properly diagnose the side effects and differentiate them from other skin disorders.
- EGFR Inhibitors-Induced Skin Disorders market dynamics are anticipated to change in the coming years due to the launch of new therapies during the forecast period (2024-2034). The high disease burden and lack of approved curative treatments are the major factors propelling the EGFR Inhibitors-Induced Skin Disorders market in the study period. The major key players include Lutris Pharma, Hoth Therapeutics, and others.
DelveInsight’s "EGFR Inhibitors-Induced Skin Disorders Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of EGFR Inhibitors-Induced Skin Disorders, historical and forecasted epidemiology as well as the EGFR Inhibitors-Induced Skin Disorders market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The EGFR Inhibitors-Induced Skin Disorders market report provides current treatment practices, emerging drugs, EGFR Inhibitors-Induced Skin Disorders market share of individual therapies, and current and forecasted EGFR Inhibitors-Induced Skin Disorders market size from 2020 to 2034, segmented by seven major markets. The report also covers current EGFR Inhibitors-Induced Skin Disorders treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
EGFR Inhibitors-Induced Skin Disorders Epidemiology
|
Segmented by:
|
|
EGFR Inhibitors-Induced Skin Disorders key companies |
|
|
EGFR Inhibitors-Induced Skin Disorders key therapies |
|
|
EGFR Inhibitors-Induced Skin Disorders Market |
Segmented by:
|
|
Analysis |
|
Study Period: 2020–2034
EGFR Inhibitors Induced Skin Disorders Understanding and Treatment Algorithm
EGFR Inhibitors Induced Skin Disorders Overview
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is frequently mutated or overexpressed in a large number of tumors such as carcinomas or glioblastoma. Inhibitors of EGFR activation have been successfully established for the therapy of some cancers and are more and more frequently being used as first or later-line therapies. Although the side effects induced by inhibitors of EGFR are less severe than those observed with classic cytotoxic chemotherapy and can usually be handled by outpatient care, they may still be a cause for dose reduction or discontinuation of treatment that can reduce the effectiveness of antitumor therapy.
EGFR Inhibitors Induced Skin Disorders Diagnosis
Diagnosing skin disorders induced by EGFR inhibitors involves a thorough evaluation by healthcare professionals, often oncologists or dermatologists. They conduct a comprehensive physical examination, scrutinizing the skin, nails, and hair for characteristic signs like acneiform rash, xerosis, pruritus, paronychia, or hair changes. Patient history, including cancer diagnosis and treatment details, is crucial to correlate symptom onset with EGFR inhibitor therapy. Differential diagnosis considers alternative causes like infections or pre-existing dermatological conditions. In uncertain cases, a skin biopsy may be warranted. Laboratory tests may be ordered to assess systemic issues.
Further details related to diagnosis will be provided in the report…
EGFR Inhibitors Induced Skin Disorders Treatment
Treating skin disorders triggered by EGFR inhibitors entails a multifaceted strategy, including topical remedies like emollients, corticosteroids, and antibiotics for rash, xerosis, and infections. Oral antibiotics and antihistamines may be prescribed for more severe cases. Systemic corticosteroids and temporary EGFR inhibitor dose adjustments might be necessary for severe reactions. Patients benefit from education on skincare and sun protection. Specialized care, including dermatologist consultation for severe cases or nail care, may be needed. A collaborative effort among oncologists, dermatologists, and support teams ensures comprehensive management, aiming to alleviate symptoms while optimizing cancer treatment outcomes.
Further details related to treatment will be provided in the report…..
EGFR Inhibitors Induced Skin Disorders Epidemiology
The EGFR Inhibitors Induced Skin Disorders epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total incident cases of indications harboring EGFR mutation (NSCLC, Colorectal cancer, Pancreatic, Head and Neck cancer, Breast cancer), incident cases of EGFR mutation in respective indications, treatable cases by EGFR inhibitors, incident cases of EGFR inhibitors-induced skin disorders, in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- EGFR is altered in 3.22% of colorectal carcinoma patients with EGFR mutation present in 2.33% of all colorectal carcinoma patients.
- Nearly 20% of patients are affected with EGFR mutation in NSCLC.
- In pancreatic ductal carcinoma, EGFR is overexpressed in 30–89% of the cases.
- In 2023, there were more than 30,000 treatable incident cases of EGFR inhibitors in the United States.
EGFR Inhibitors Induced Skin Disorders Drug Chapters
The drug chapter segment of the EGFR Inhibitors Induced Skin Disorders report encloses a detailed analysis of the late-stage (Phase III) and mid-stage (Phase II/III and Phase II) pipeline drugs. The current key players include Lutris Pharma (LUT014), Hoth Therapeutics (HT-001), and others. The drug chapter also helps understand the EGFR Inhibitors-Induced Skin Disorders clinical trial details, pharmacological action, agreements and collaborations, approval, and patent details, and the latest news and press releases.
Emerging Drugs
LUT014: Lutris Pharma
LUT014 is a topical B-RAF inhibitor, first-in-class, small molecule. Lutris Pharma is conducting a Phase II, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of LUT014 in metastatic colorectal cancer patients with EGFR Inhibitors-induced acneiform lesions. The Phase I study demonstrated the first evidence of treatment effectiveness by reducing the severity of acneiform lesions and improving patient quality of life. Promising preliminary data from the Phase II study may point to a dose-response and the high dose success rate achieved demonstrates the efficacy of LUT014.
HT-001: Hoth Therapeutics
It is a topical formulation being developed by Hoth Therapeutics and is currently under development to treat patients with mild to moderate rash and skin disorders associated with initial and repeat courses of tyrosine kinase inhibitor/epidermal growth factor receptor inhibitor therapy. The Phase IIa CLEER clinical trial is currently underway to evaluate the safety and efficacy of HT-001 in patients with rash and skin disorders associated with EGFR inhibitor therapy. In January 2023, the US FDA accepted an IND application for HT-001 for the treatment of rash and skin disorders associated with EGFR inhibitor therapy.
Detailed emerging therapies assessment will be provided in the final report.
EGFR Inhibitors-Induced Skin Disorders Market Outlook
The treatment of EGFR inhibitor related skin reactions is not standardized and is based on local practice and mainly derived from personal, albeit extensive, experience. Currently, there is no approved therapy specific for anti-EGFR-induced skin diseases in the US, EU4 the UK, and Japan. The off-label therapeutic options used for anti-EGFR-induced skin diseases are sunscreen, emollients and soap substitutes, antibiotics, antihistamines, topical steroids, and cosmetics. In contrast to acne vulgaris, topical antibiotic treatment with erythromycin, metronidazole, or nadifloxacin twice daily is recommended for early-stage and low-grade papulopustular skin reactions. Skin moisturizer and urea- or polidocanol-containing lotions are used to soothe. The rise in the patient pool of EGFR Inhibitors-Induced Skin Disorders is due to a hike in access to EGFR inhibitors and an increase in the incidence of EGFR mutated cancer cases, the expected launch of emerging therapies will drive the EGFR inhibitors-induced skin disorders treatment market in the forecasted period (2024–2034).
Detailed market assessment will be provided in the final report.
EGFR Inhibitors-Induced Skin Disorders Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034. The landscape of EGFR Inhibitors-Induced Skin Disorders treatment has experienced a transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care.
EGFR Inhibitors-Induced Skin Disorders Pipeline Development Activities
The report provides insights into therapeutic candidates in different stages. It also analyzes key players involved in developing targeted therapeutics. Companies such as Lutris Pharma and Hoth Therapeutics actively engage in late and mid-stage research and development efforts for EGFR Inhibitors Induced Skin Disorders. The pipeline of EGFR Inhibitors-Induced Skin Disorders possesses potential drugs. However, there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for EGFR Inhibitors Induced Skin Disorders emerging therapy.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the EGFR Inhibitors Induced Skin Disorders evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including MDs, Hematologists, ophthalmologists, and others.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. The opinion helps understand and validate current and emerging therapy treatment patterns or EGFR Inhibitors-Induced Skin Disorders market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Market Access and Reimbursement
Referring patients with EGFR Inhibitors-Induced Skin Disorders to a dermatologist specialist is common practice, but for patients with EGFR Inhibitors-Induced Skin Disorders, dermatologists may feel that their options are limited. These patients require frequent monitoring to watch for the development of EGFR Inhibitors-Induced Skin Disorders.
The United States drug pricing regime is complex given its multi-payer model. It is currently undergoing significant changes, with recent federal legislation, such as the Prescription Drug Pricing Reform provisions of the Inflation Reduction Act, significantly altering the pricing regime under certain federal programs.
The US health care system includes both private and public health insurance coverage. Whether a drug product is covered, and at what price, is determined by each payer’s coverage, coding, and payment criteria for health insurance plans. The largest government-funded programs are Medicare and Medicaid. Private plans, which cover far more Americans than public plans, have more flexibility to make coverage and reimbursement determinations.
Detailed market access and reimbursement assessment will be provided in the final report.
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of EGFR Inhibitors Induced Skin Disorders, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the EGFR Inhibitors Induced Skin Disorders market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive EGFR Inhibitors-Induced Skin Disorders.
EGFR Inhibitors-Induced Skin Disorders Report Insights
- Patient Population
- Therapeutic Approaches
- EGFR Inhibitors Induced Skin Disorders Pipeline Analysis
- EGFR Inhibitors Induced Skin Disorders Market Size and Trends
- Existing and Future Market Opportunity
EGFR Inhibitors-Induced Skin Disorders Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- EGFR Inhibitors Induced Skin Disorders Epidemiology Segmentation
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
EGFR Inhibitors-Induced Skin Disorders Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Reasons to Buy
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving EGFR Inhibitors Induced Skin Disorders.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential current and emerging therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.