Facioscapulohumeral Muscular Dystrophy Market
- Facioscapulohumeral Muscular Dystrophy (FSHD) is a genetically acquired disease that leads to progressive muscle weakness and severely decreased functional capacity in affected individuals. FSHD is estimated to be the second most prevalent dystrophy after Duchenne muscular dystrophy.
- As per DelveInsight analysis, the US showed the highest number of Facioscapulohumeral Muscular Dystrophy Prevalent Cases, accounting for nearly 42% of the total Facioscapulohumeral Muscular Dystrophy Prevalent Cases in the 7MM in 2023.
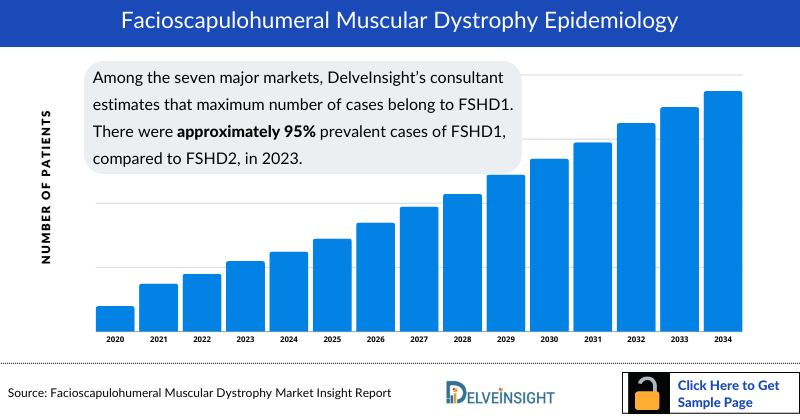
- In the seven major markets, FSHD Type 1 accounted for approximately 95% of total Facioscapulohumeral Muscular Dystrophy Prevalent Cases. FSHD type 1 (FSHD1) is dominantly inherited, while individuals must inherit one defective copy of a segment of DNA from each parent in order to have FSHD Type 2.
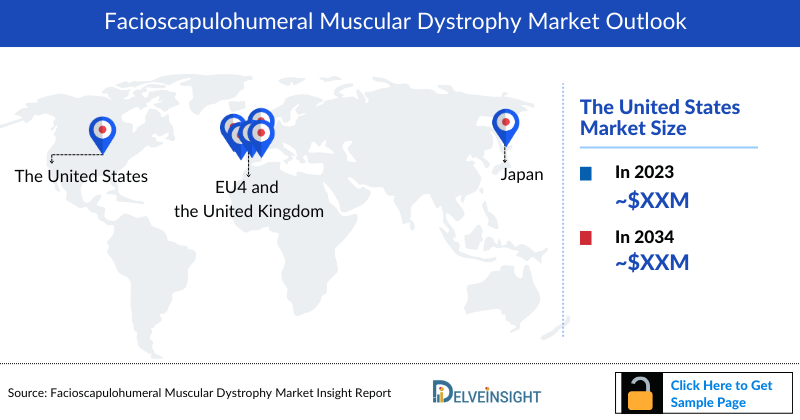
- The United States accounts for the largest market share (around 80%) of Facioscapulohumeral Muscular Dystrophy Market, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- There are currently no disease-modifying treatments for FSHD; supportive care is the mainstay of managing FSHD.
- Currently, few therapies are in development for FSHD. Some of the most prominent ones include losmapimod (Fulcrum Therapeutics), RO7204239/GYM-329/RG-6237 (Hoffmann-La Roche), and others.
- Fulcrum Therapeutics expects to report topline data of REACH, the Phase III Facioscapulohumeral Muscular Dystrophy Clinical Trials evaluating losmapimod in patients with FSHD in the fourth quarter of 2024.
- Growing numbers of Facioscapulohumeral Muscular Dystrophy Companies and academic laboratories are pressing forward with early-stage drug development efforts. Various organizations, such as the FSHD Society, Molecular Dystrophy Association, and The Chris Carrino Foundation for FSHD, solve FSHD, and others are working to accelerate research leading to treatments and a cure.
Request for unlocking the sample page of the "Facioscapulohumeral Muscular Dystrophy Treatment Market"
DelveInsight's “Facioscapulohumeral Muscular Dystrophy Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of facioscapulohumeral muscular dystrophy, historical and forecasted epidemiology as well as the facioscapulohumeral muscular dystrophy market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Facioscapulohumeral Muscular Dystrophy Treatment Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM facioscapulohumeral muscular dystrophy market size from 2020 to 2034. The report also covers current facioscapulohumeral muscular dystrophy treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Facioscapulohumeral Muscular Dystrophy Segmentation |
Segmented by: Region
|
|
Facioscapulohumeral Muscular Dystrophy Companies |
|
|
Facioscapulohumeral Muscular Dystrophy Drugs |
|
|
Facioscapulohumeral Muscular Dystrophy Market |
Segmented by:
|
|
Analysis |
|
Facioscapulohumeral Muscular Dystrophy Treatment Market
Facioscapulohumeral muscular dystrophy (FSHD) is one of the most common forms of muscular dystrophy, with a distinctive pattern of skeletal muscle weakness and a wide spectrum of disease severity. It is a disorder characterized by muscle weakness and muscle wasting (atrophy). FSHD is most typically characterized by relatively slow disease progression and is usually inherited as an autosomal dominant genetic condition. Moreover, although FSHD does not typically impact lifespan, some patients may develop life-threatening respiratory muscle complications.
The diagnostic criteria of FSHD have evolved from a cluster of clinical symptoms and laboratory findings to genetic analysis of suspected cases. While genetic testing is the current gold standard in evaluating patients, equivocal presentations are aided by EMG studies, MRI, laboratory tests, and muscle biopsies. The facioscapulohumeral muscular dystrophy therapeutics market report provides an overview of facioscapulohumeral muscular dystrophy pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Facioscapulohumeral Muscular Dystrophy Treatment
Currently, there are no curative genetic or pharmaceutical treatments for this disease. The mainstay of management is care directed at the symptomatic impairments to maximize functional abilities and improve patients’ QoL. In cases of severe pathology, physical therapy alone may not be enough to correct functional limitations. Assistive devices are, therefore, useful and can be tailored to each patient’s specific needs. Foot drop can be partially corrected using ankle-foot orthoses or in combination with knee-ankle-foot orthoses. Surgical scapular fixation might be offered cautiously to selected patients after careful consideration of the overall muscle impairment in the involved arm, assessment of potential gain in the range of motion by manual fixation of the scapula, the patient’s rate of disease progression, and the potential adverse consequences of surgery and prolonged postsurgical bracing.
Facioscapulohumeral Muscular Dystrophy Epidemiology
The facioscapulohumeral muscular dystrophy epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The facioscapulohumeral muscular dystrophy epidemiology is segmented with detailed insights into Total Facioscapulohumeral Muscular Dystrophy Prevalence Cases, Total Facioscapulohumeral Muscular Dystrophy Diagnosed Cases, Facioscapulohumeral Muscular Dystrophy Type-specific Cases, Facioscapulohumeral Muscular Dystrophy Gender-specific Cases, Facioscapulohumeral Muscular Dystrophy Age-specific Cases, Facioscapulohumeral Muscular Dystrophy Severity-specific Cases, and Total Facioscapulohumeral Muscular Dystrophy Treated Cases.
- Among the seven major markets, DelveInsight’s consultant estimates that the maximum number of cases belong to FSHD1. There were approximately 95% prevalent cases of FSHD1, compared to FSHD2, in 2023.
- The Facioscapulohumeral Muscular Dystrophy gender-specific cases were slightly more in males than females in the United States in 2023. Approximately 70% of the total prevalent FSHD cases fall within the Ricci severity score (4-10).
- The United States accounted for approximately 40% of total Facioscapulohumeral Muscular Dystrophy Prevalence Cases recorded in the seven major markets.
- Based on the age-specific prevalence, it has been shown that the 50 years and above age group had the highest Facioscapulohumeral Muscular Dystrophy Prevalence across the seven major markets.
- Among the EU4 and the UK, Germany recorded the highest number of Facioscapulohumeral Muscular Dystrophy Prevalent Cases in 2023.
Recent Developments in the Facioscapulohumeral Muscular Dystrophy Market Landscape
- In April 2025, Epicrispr Biotechnologies announced FDA clearance of its IND application for EPI-321, a novel epigenetic therapy targeting facioscapulohumeral muscular dystrophy (FSHD).
Facioscapulohumeral Muscular Dystrophy Drugs Market Chapters
The drug chapter segment of the facioscapulohumeral muscular dystrophy market report encloses a detailed analysis of facioscapulohumeral muscular dystrophy marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the facioscapulohumeral muscular dystrophy pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Facioscapulohumeral Muscular Dystrophy Emerging Drugs
- Losmapimod: Fulcrum Therapeutics/GSK
Losmapimod is an investigational, selective p38α/β mitogen-activated protein kinase (MAPK) inhibitor. Utilizing its internal product engine, Fulcrum discovered that inhibition of p38α/β reduced expression of the DUX4 gene in muscle cells derived from patients with FSHD. Currently, the molecule is in Phase III trial (NCT05397470) to treat patients with genetically confirmed diagnoses of FSHD 1 or FSHD 2. Fulcrum anticipates reporting top-line data from the REACH Phase III clinical trial assessing losmapimod in FSHD Patients in the fourth quarter of 2024.
- GYM329 (RO7204239/RG 6237): Roche
GYM329 is an investigational anti-myostatin antibody designed to target skeletal muscles, potentially increasing their size and growth. Currently, it is being investigated under Phase II trial in participants with FSHD. Moreover, this trial is expected to be completed May 2025. The company expects to start the regulatory submissions after 2027.
|
Drugs |
Company |
MOA |
ROA |
Phase |
|
Losmapimod |
Fulcrum Therapeutics/GSK
|
p38α/β MAPK inhibitor (P38 mitogen-activated protein kinase inhibitors) |
Oral |
III |
|
GYM329 (RO7204239/ RG 6237) |
Roche |
Myostatin inhibitors |
SC |
II |
Facioscapulohumeral Muscular Dystrophy Market Outlook
Facioscapulohumeral Muscular Dystrophy Companies, such as Fulcrum Therapeutics/GSK, Roche, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of facioscapulohumeral muscular dystrophy.
- DelveInsight estimates show that the US accounted for the highest Facioscapulohumeral Muscular Dystrophy market size, with nearly 80% of the FSHD Market Share as compared to the EU4 and the UK and Japan
- During the forecast period (2024–2034), Facioscapulohumeral Muscular Dystrophy Pipeline candidates such as losmapimod (Fulcrum Therapeutics), and RO7204239/GYM-329/RG-6237 (Hoffmann-La Roche) are expected to drive the rise in the Facioscapulohumeral Muscular Dystrophy market size.
- Japan accounts for the second highest Facioscapulohumeral Muscular Dystrophy market size in the 7MM during the forecast period 2024–2034.
- Among the seven major markets, Cocodamol generated highest revenue, followed by Ibuprofen and Tramadol, in 2023.
- Facioscapulohumeral Muscular Dystrophy Market growth is expected to be driven by the entry of novel therapies with better clinical profiles, an increase in market penetration of advanced therapies, an upsurge in research and development, an enriched understanding of the disease, and imminent launches of the drugs.
Facioscapulohumeral Muscular Dystrophy Drugs Uptake
This section focuses on the uptake rate of potential Facioscapulohumeral Muscular Dystrophy drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Facioscapulohumeral Muscular Dystrophy Activities
The Facioscapulohumeral Muscular Dystrophy Therapeutics Market report provides insights into different therapeutic candidates in the Phase III and Phase II stages. It also analyzes key Facioscapulohumeral Muscular Dystrophy Companies involved in developing targeted therapeutics.
Facioscapulohumeral Muscular Dystrophy Pipeline Development Activities
The Facioscapulohumeral Muscular Dystrophy Therapeutics Market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for facioscapulohumeral muscular dystrophy emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as University of Iowa Hospitals & Clinics, Queen Square Centre for Neuromuscular Diseases, University Hospital Essen, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of facioscapulohumeral muscular dystrophy. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
Region |
KOL Views |
|
United States |
“Based upon a broad array of evidence, DUX4 is regarded as a validated target for therapy development in FSHD. Although this target is deemed a developmental transcription factor, FSHD1 and FSHD2 involve upregulated expression of DUX4 and aberrant activation of its normally early, developmentally regulated transcriptional program. More recent evidence of target validity comes from the positive correlation between local DUX4 activation and muscle pathology detected by MRI. Yet activation of DUX4 in affected skeletal muscles occurs in difficult-to-capture transient bursts, raising issues of its potential biomarker value and directing efforts toward targets up- or downstream of DUX4.” |
|
Japan |
“There are exciting new developments in FSHD genetic testing. PerkinElmer Genomic is offering a genetic test for FSHD. Other companies and groups are likely to follow suit. This is the first major innovation in FSHD genetic testing in nearly three decades to “reach the clinic”—available for the doctor to order. This development has several advantages for patients and families: this new test for FSHD Type 1 is expected to be two to three times less expensive than current alternatives. The company offers a comprehensive test for 132 other neuromuscular conditions, including FSHD Type 2 and the FSHD Type 1 assay.” |
Facioscapulohumeral Muscular Dystrophy Drugs Market: Qualitative Analysis
Facioscapulohumeral Muscular Dystrophy Therapeutics Market Access and Reimbursement
Facioscapulohumeral Muscular Dystrophy Treatment Market Report Scope
- The Facioscapulohumeral Muscular Dystrophy treatment market report covers a segment of key events, an executive summary, descriptive overview of facioscapulohumeral muscular dystrophy, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the facioscapulohumeral muscular dystrophy treatment market, historical and forecasted Facioscapulohumeral Muscular Dystrophy treatment market size, Facioscapulohumeral Muscular Dystrophy drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Facioscapulohumeral Muscular Dystrophy treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM facioscapulohumeral muscular dystrophy drugs market.
Facioscapulohumeral Muscular Dystrophy Treatment Market Report Insights
- Patient-based Facioscapulohumeral Muscular Dystrophy Market Forecasting
- Therapeutic Approaches
- Facioscapulohumeral Muscular Dystrophy Pipeline Analysis
- Facioscapulohumeral Muscular Dystrophy Market Size
- Facioscapulohumeral Muscular Dystrophy Market Trends
- Existing and Future Facioscapulohumeral Muscular Dystrophy Drugs Market Opportunity
Facioscapulohumeral Muscular Dystrophy Treatment Market Report Key Strengths
- 11-year Facioscapulohumeral Muscular Dystrophy Market Forecast
- 7MM Coverage
- Facioscapulohumeral Muscular Dystrophy Epidemiology Segmentation
- Inclusion of Country Specific Treatment Guidelines
- KOL’s Feedback on Approved and Emerging Therapies
- Key Cross Competition
- Conjoint Analysis
- Facioscapulohumeral Muscular Dystrophy Drugs Uptake
- Facioscapulohumeral Muscular Dystrophy Market Forecast Assumptions
Facioscapulohumeral Muscular Dystrophy Treatment Market Report Assessment
- Current Facioscapulohumeral Muscular Dystrophy Treatment Market Practices
- Facioscapulohumeral Muscular Dystrophy Unmet Needs
- Facioscapulohumeral Muscular Dystrophy Pipeline Product Profiles
- Facioscapulohumeral Muscular Dystrophy Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- What is the growth rate of the 7MM facioscapulohumeral muscular dystrophy treatment market?
- What was the facioscapulohumeral muscular dystrophy treatment market size, the Facioscapulohumeral Muscular Dystrophy drugs market size by therapies, the Facioscapulohumeral Muscular Dystrophy drugs market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of facioscapulohumeral muscular dystrophy?
- How many FSHD companies are developing therapies for the treatment of facioscapulohumeral muscular dystrophy?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
- The Facioscapulohumeral Muscular Dystrophy Treatment Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the facioscapulohumeral muscular dystrophy drugs market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Facioscapulohumeral Muscular Dystrophy market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Facioscapulohumeral Muscular Dystrophy drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Facioscapulohumeral Muscular Dystrophy drugs market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles

.jpg)
.jpg)
.jpg)



