Friedreich's Ataxia Market
- The Friedreich’s Ataxia market size is projected to experience growth at a consistent CAGR during the forecast period (2024-2034). The growth in market revenue is primarily driven by the increasing research and development activities and the development of novel treatments.
- Friedreich's Ataxia is one of the most common forms of inherited ataxia, with the condition typically manifesting in childhood or adolescence and progressing over time. Despite its rarity, it presents significant challenges due to its progressive nature and debilitating symptoms.
- The current treatment regime is restrictive with only one approved product, and there is a critical need for disease-modifying therapies that can address the underlying genetic defect and provide meaningful improvements in the quality of life for individuals.
- Increased focus on drug development, advances in genetics and genomics, and a growing market for precision medicine, among other factors may change the market scenario in times to come. Several companies are in the market race as their drugs are in the pipeline to excel in the coming years like vatiquinone (PTC Therapeutics), MIB-626 (Metro International Biotech), leriglitazone (Minoryx Therapeutics), and others.
Request for unlocking the CAGR of the "Friedreich’s Ataxia Drugs Market"
DelveInsight’s comprehensive report titled “Friedreich’s Ataxia Drugs Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Friedreich’s Ataxia. The report presents historical and projected epidemiological data covering total diagnosed prevalent cases of Friedreich’s Ataxia, prevalent cases of Friedreich’s Ataxia based on onset types, and treated cases of Friedreich’s Ataxia. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The Friedreich's Ataxia Drugs Market Report analyzes the existing treatment practices and Friedreich's Ataxia Unmet Medical requirements. It evaluates the Friedreich's Ataxia drugs market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the Friedreich's Ataxia treatment market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Friedreich’s Ataxia Epidemiology |
|
|
Friedreich’s Ataxia Drugs Market |
|
|
Friedreich's Ataxia Drugs Market Analysis |
|
|
Friedreich’s Ataxia Companies |
|
|
Future opportunity |
The development of breakthrough therapies, disease-modifying treatments, increased awareness and access to care, advancements in genetic research, and personalized medicine may open up new avenues for targeted treatments, providing further opportunities for market growth. |
Friedreich’s Ataxia Treatment Market: Overview
Friedreich’s Ataxia is a rare inherited disease that causes progressive nervous system damage and movement problems. It usually begins in childhood and leads to impaired muscle coordination (ataxia) that worsens over time. It is caused due to a defect (mutation) in a gene labeled FXN, which carries the genetic code for a protein called frataxin. Therefore, the individuals who inherit two defective copies of the gene, one from each parent, will develop the disease.
The inherited disorder is characterized by two subtypes: LOFA and VLOFA. For LOFA, the age of onset is between the ages of 26 and 39 years, and for VLOFA the age of onset is after the age of 40 years. Friedreich’s Ataxia symptoms include difficulty walking and poor balance. The symptoms typically begin between the ages of 5 and 15 years. Usually, the first neurological symptom is difficulty walking and poor balance. Another early sign of the disease is slowness and slurring of speech (dysarthria). With time speech becomes hesitant and jerky (referred to as “scanning of speech”). The difficulty coordinating movement can affect all of the muscles. It gradually worsens and slowly spreads to the arms and the trunk.
Friedreich’s Ataxia Diagnosis and Treatment Algorithm
Friedreich's ataxia diagnosis requires a careful clinical examination, that includes a thorough physical exam, looking for balance difficulty, loss of joint sensation (proprioception), absence of reflexes, and signs of neurological problems. Moreover, genetic testing now provides a conclusive diagnosis. Treatments for Friedreich's ataxia have targeted specific symptoms of the disease rather than the underlying cause, and to a greater degree, these therapeutic options still make up the gold standard for Friedreich's ataxia care. There is no cure for the disease, but there are ways to manage the condition and improve the quality of life for those living with Friedreich's ataxia
There was no approved therapy for Friedreich’s ataxia before February 2023, when Reata Pharmaceuticals’ SKYCLARYS (omaveloxolone) was approved by the US FDA. Friedreich's ataxia remained devoid of any cure or efficacious treatment options, however, the approval of SKYCLARYS (omaveloxolone) marked a pivotal moment in the medical landscape, offering newfound hope to individuals battling with the challenges posed by Friedreich's ataxia.
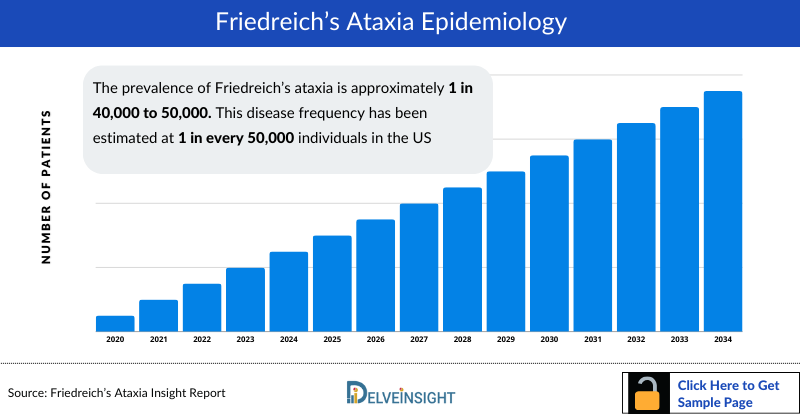
Friedreich’s Ataxia Epidemiology
The epidemiology section of Friedreich’s Ataxia market size report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The prevalent patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of Friedreich’s Ataxia. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- As per research findings, Friedreich's ataxia is the most common hereditary ataxia that appears to affect males and females equally. The prevalence of Friedreich's ataxia is approximately 1 in 40,000 to 50,000. This disease frequency has been estimated at 1 in every 50,000 individuals in the United States.
- A study reported the analyzed Friedreich's ataxia prevalence which is the most common of the inherited ataxias across most of Europe. Its prevalence is highest in Western Europe, with more than 1 case per 30,000 individuals.
- According to the research study, the prevalence of Friedreich’s ataxia in Japan is 13.1 per 100,000 individuals.
Unlock comprehensive insights! Click Here to Purchase the Full Report @ Friedreich’s Ataxia Prevalence
Friedreich’s Ataxia Market Outlook
The Friedreich's ataxia treatment market goal primarily focuses on managing symptoms, slowing down the progression of the disease, and improving the quality of life for patients. This often involves a multidisciplinary approach, including physical therapy, occupational therapy, speech therapy, and medications to manage symptoms such as slurred speech, muscle stiffness, loss of reflexes, and fatigue. Additionally, research efforts are ongoing to develop therapies that target the underlying genetic cause of the disease, with the ultimate aim of finding a cure.
Currently, there is no specific medication approved to treat the underlying cause of Friedreich's ataxia, which is a genetic disorder. However, medications may be prescribed to help manage symptoms and improve the quality of life for individuals with the condition. Physical therapy is often recommended to manage the disease, as it helps improve strength, coordination, and balance, as well as alleviate symptoms such as muscle stiffness and unsteady gait.
- In February 2023, the US FDA approved the use of SKYCLARYS (omaveloxolone) to treat Friedreich’s Ataxia. It is a significant milestone as the first and only FDA-approved treatment for Friedreich’s Ataxia in individuals aged 16 years old and above. With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, Friedreich’s Ataxia market in the 7MM is expected to change significantly during the study period 2020–2034.
Friedreich’s Ataxia Recent Developments
- In October 2025, Lexeo Therapeutics announced FDA support for pooling Phase I/II data with pivotal trial results to pursue Accelerated Approval for LX2006 in Friedreich ataxia (FA) cardiomyopathy. Interim data show sustained improvements in cardiac and neurological outcomes, including an 18% reduction in left ventricular mass index (LVMI) at 6 months and 23% at 12 months—surpassing FDA targets—and meaningful gains on the modified Friedreich Ataxia Rating Scale (mFARS), suggesting slowed disease progression.
- In October 2025, Lexeo Therapeutics shared updates on the Accelerated Approval pathway for LX2006 in Friedreich ataxia cardiomyopathy, along with new interim Phase I/II data.
- In June 2025, Larimar Therapeutics announced FDA safety database recommendations and an updated BLA submission timeline for its Friedreich’s Ataxia (FA) treatment, incorporating adult and pediatric safety data under the START program.
- In January 2025, Solid Biosciences Inc. announced that the FDA approved its Investigational New Drug (IND) application for SGT-212, a gene therapy for Friedreich’s ataxia, a progressive disease causing nervous system damage and cardiac dysfunction due to low frataxin production.
Friedreich’s Ataxia Drug Chapters
Friedreich’s Ataxia Marketed Drugs
- SKYCLARYS (omaveloxolone): Reata Pharmaceuticals
SKYCLARYS, developed by Reata Pharmaceuticals, is a groundbreaking therapy that Skyclarys is designed to activate nuclear factor erythroid 2-related factor 2 (NrF2), a type of protein called a transcription factor whose signaling is impaired in FA patients. NrF2 works to activate genes that promote mitochondrial function, boost antioxidant responses, and prevent inflammation.
Note: Detailed marketed therapies assessment will be provided in the final report.
Friedreich’s Ataxia Emerging Drugs
The Friedreich’s Ataxia drugs market is expected to experience gradual changes, mainly due to the limited availability of emerging therapies in this area. Key market players, including Vatiquinone, Lexeo Therapeutics, and others, have demonstrated a keen interest in this rare condition and are actively pursuing the development of potential treatments.
- Vatiquinone: PTC Therapeutics
Vatiquinone is an investigational small molecule that inhibits 15-Lipoxygenase, an enzyme that is a key regulator of the oxidative stress and inflammation response pathways that underpin neurological disease pathology. In March 2014, the US FDA granted fast-track designation to Vatiquinone for the treatment of Friedreich's Ataxia. It has also received orphan drug designation from the US FDA for the treatment of Friedreich's Ataxia. The company recently announced results from the Phase III MOVE-FA study evaluating vatiquinone (PTC743) in children and young adults with Friedreich's ataxia and held a meeting with the US FDA. Based on the US FDA feedback from the Live Type C Meeting, the company plans to submit an NDA for vatiquinone for the treatment of Friedreich's ataxia in late 2024.
In addition, LX2006 (Lexeo Therapeutics) and elamipretide (Stealth BioTherapeutics) are in the early phase of clinical development for the treatment of Friedreich's ataxia.
Friedreich’s Ataxia Drugs Market Segmentation
DelveInsight’s ‘Friedreich’s Ataxia Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Friedreich’s Ataxia Treatment Market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future Friedreich's Ataxia Market Share of all therapies.
Friedreich’s Ataxia Therapeutics Therapeutics Market Size by Countries
The Friedreich’s Ataxia treatment market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM Friedreich’s Ataxia market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Friedreich’s Ataxia Therapeutics Market Size by Therapies
Friedreich’s Ataxia Therapeutics Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is Vatiquinone under the developmental pipeline PTC Therapeutics.
Friedreich’s Ataxia Drugs Uptake
This section focuses on the sales uptake of potential Friedreich’s Ataxia drugs that have recently been launched or are anticipated to be launched in the Friedreich’s Ataxia market between 2020 and 2034. It estimates the market penetration of Friedreich’s Ataxia drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in Friedreich’s Ataxia Therapeutics Market. The emerging Friedreich’s Ataxia therapies are analyzed based on various attributes such as safety and efficacy in randomized Friedreich's Ataxia clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Friedreich’s Ataxia Therapeutics Market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Friedreich’s Ataxia.
Friedreich’s Ataxia Market Access and Reimbursement
DelveInsight’s ‘Friedreich’s Ataxia Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Friedreich’s Ataxia. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Friedreich’s Ataxia market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Friedreich’s Ataxia domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Friedreich’s Ataxia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and Friedreich’s Ataxia unmet needs.
Friedreich’s Ataxia: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Boston Children’s Hospital in the US, CIBER de Enfermedades Cardiovasculares (CIBERCV) in Spain, Oita University, Yufu in Japan, University Children’s Hospital, Magdeburg, in Germany, and organizations like Friedreich’s Ataxia Research Foundation, International Friedreich’s Ataxia Registry, among others.
“Many patients experience the heavy burden of Friedreich's Ataxia attacks in their daily lives. Now, the Friedreich's Ataxia community has a new treatment option, clinically proven to significantly reduce the frequency of Friedreich's Ataxia attacks.”
Note: Detailed assessment of KOL Views will be provided in the full report on Friedreich’s Ataxia.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of Friedreich’s Ataxia Therapeutics Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Friedreich’s Ataxia Pipeline Development Activities
The Friedreich’s Ataxia pipeline segment offers an analysis of therapeutic candidates in Phase II and III stages and examines Friedreich’s Ataxia companies involved in developing targeted therapeutics for Friedreich’s Ataxia. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The Friedreich’s Ataxia pipeline segment covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Friedreich’s Ataxia therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Friedreich’s Ataxia Treatment Drugs
Friedreich’s Ataxia Market Forecast Report Insights
- Patient-based Friedreich’s Ataxia Market Forecasting
- Therapeutic Approaches
- Friedreich’s Ataxia Pipeline Analysis
- Friedreich’s Ataxia Market Size
- Friedreich’s Ataxia Market Trends
- Friedreich’s Ataxia Drugs Market Opportunities
- Impact of Upcoming Therapies
Friedreich’s Ataxia Market Forecast Report Key Strengths
- 11 Years Friedreich’s Ataxia Market Forecast
- The 7MM Coverage
- Friedreich’s Ataxia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Friedreich’s Ataxia Drugs Market
- Friedreich’s Ataxia Drugs Uptake
Friedreich’s Ataxia Treatment Market Report Assessment
- Current Friedreich’s Ataxia Treatment Market Practices
- Friedreich’s Ataxia Unmet Needs
- Friedreich’s Ataxia Pipeline Product Profiles
- Friedreich’s Ataxia Drugs Market Attractiveness
Key Questions
- How common is Friedreich’s Ataxia?
- What are the key findings of Friedreich’s Ataxia epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Friedreich’s Ataxia?
- What are the disease risk, burden, and Friedreich’s Ataxia unmet needs?
- At what CAGR is Friedreich’s Ataxia drugs market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact Friedreich’s Ataxia market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Friedreich’s Ataxia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many companies are currently developing therapies for the Friedreich’s Ataxia treatment?
Access Exclusive Data Now! Click here to Read More about the Related Articles:-