HER2+ Gastric Cancer Market Summary
- The HER2+ Gastric Cancer market size in the 7MM is expected to grow from USD 702 million in 2025 to USD 2,318 million in 2034.
- The HER2+ Gastric Cancer market is projected to grow at a CAGR of 14.20% by 2034 in leading countries like US, EU4, UK and Japan.
HER2+ Gastric Cancer Market and Epidemiology Analysis
- Gastric cancer represents the fifth most prevalent malignant tumor, and the incidence of gastric cancer varies geographically across the globe. The highest incidence rates are observed in Eastern Asia and Eastern Europe. Japan has the highest rates of gastric cancer among the seven major markets, with around 129,500 cases, in 2024.
- Despite the high incidence of gastric cancer, most patients are unfortunately diagnosed at advanced stages with poor prognoses due to the absence of distinguishing clinical indicators. Notably, in Western countries such as the US, Germany, Italy, France, and the UK, a higher proportion of cases are diagnosed at late stages (Stage IV), in contrast to that in Japan, the majority of cases are diagnosed at an early stage (Stage I). At the time of diagnosis, metastatic disease is present in about 35% of patients in the United States.
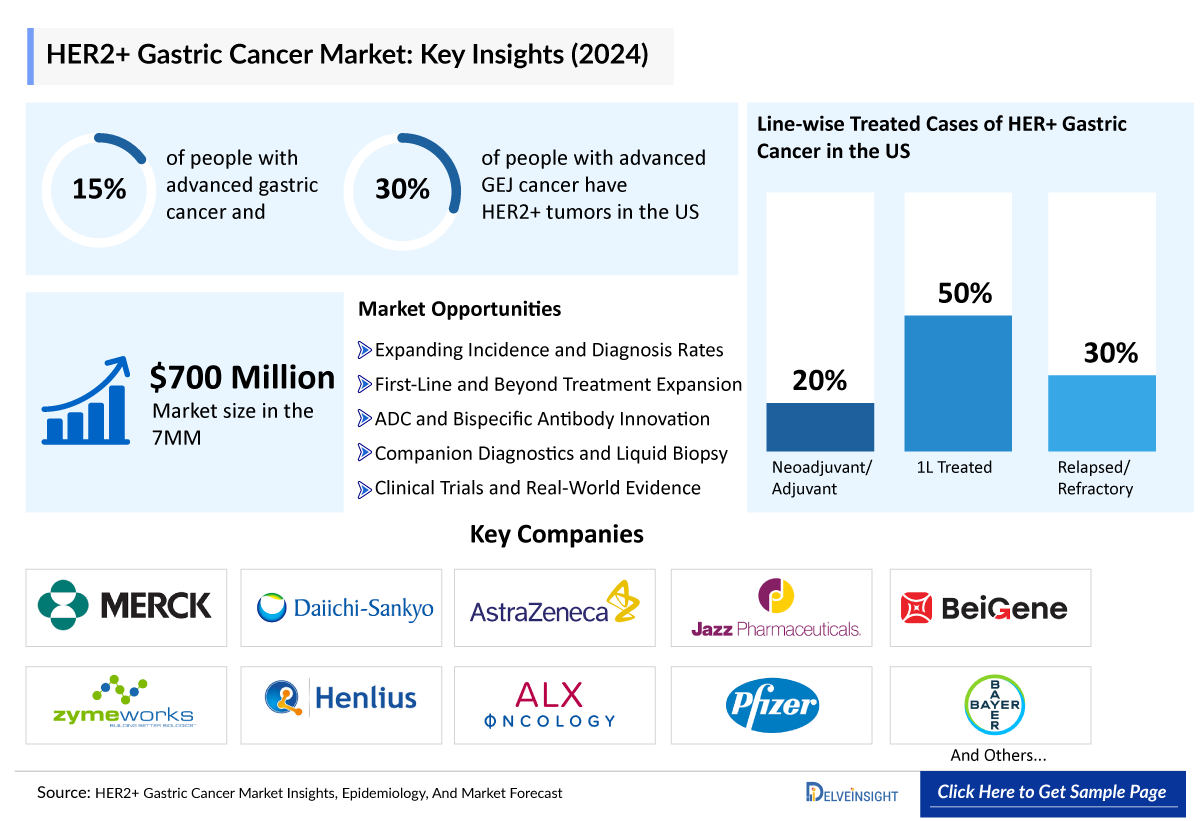
- In the United States, about 15% of people with advanced gastric cancer and 30% of people with advanced GEJ cancer have HER2+ tumors.
- In 2024, the total number of incident cases of HER2+ gastric cancer in the US was approximately 6,650, whereas Japan reported the highest incidence, with around 27,000 cases.
- In 2024, the market size of HER2+ gastric cancer was highest in the US among the 7MM, accounting for approximately USD ~300 million, which is further expected to increase by 2034.
- Historically, platinum-based chemotherapy has been the standard of care for patients with early-stage gastric cancer, highlighting the need for more effective treatment options. The mainstay of treatment for these patients includes gastrectomy (curative surgical resection) along with chemotherapy, targeted-based therapies, and other treatment approaches.
- Daiichi Sankyo and AstraZeneca’s ENHERTU (trastuzumab deruxtecan) is the only approved therapy for the treatment of advanced or metastatic HER2+ gastric cancer for 2L therapy. The drug received accelerated approval in January 2021. Along with ENHERTU (trastuzumab deruxtecan), KEYTRUDA (pembrolizumab) and HERCEPTIN (trastuzumab) are approved for HER2+ gastric cancer for 1L therapy.
- Representing a significant advancement in the 2L treatment of gastric cancer with superior outcomes compared to traditional chemotherapy, ENHERTU is currently being evaluated in the 1L setting for HER2+ gastric cancer.
- Pharmaceutical companies developing therapies for treating HER2+ gastric cancer include ALX Oncology (Evorpacept), Jazz Pharmaceuticals, BeiGene, and Zymeworks (ZIIHERA), Enliven Therapeutics (ELVN-002), Ambrx and NovoCodex (ARX788), AstraZeneca (Rilvegostomig), Shanghai Henlius Biotech and AbClon (HLX22), Bayer (BAY2927088), KLUS Pharma (A166), Pfizer (TUKYSA), Bayer (BAY2701439), and others with their candidates in different stages of clinical development.
HER2+ Gastric Cancer Market Size and Forecasts
- 2025 Market Size: USD 702 million in 2025
- 2034 Projected Market Size: USD 2,318 million in 2034
- Growth Rate (2025-2032): 14.20% CAGR
- Largest Market: United States
DelveInsight's ‘HER2+ Gastric Cancer (including GEJ) – Market Insights, Epidemiology and Market Forecast – 2034’ report delivers an in-depth understanding of the HER2+ gastric cancer (including GEJ), historical and forecasted epidemiology as well as the HER2+ gastric cancer (including GEJ) market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
HER2+ gastric cancer (including GEJ) market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted the 7MM HER2+ gastric cancer (including GEJ) market size from 2020 to 2034. The report also covers current HER2+ gastric cancer (including GEJ) treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
HER2+ Gastric Cancer (including GEJ) Disease Understanding
HER2+ Gastric Cancer (including GEJ) Overview
Gastric cancer represents the fifth most prevalent malignant tumor and the fourth leading cause of cancer-associated mortality on a global scale.
HER2, also referred to as HER2/neu or ERBB2, is present in about 15–30% of Gastric Cancer (including Gastroesophageal Junction [GEJ]). HER2 is encoded on chromosome 17q21 and is part of the EGFR family, which also includes EGFR/HER1, HER3, and HER4. The ligand of the tyrosine kinase receptor is still not identified. HER2 forms either homodimers or heterodimers with other members of the EGFR family receptors. HER2 homodimers can lead to ligand-independent activation in case of HER2 overexpression. In gastric cancer, ERBB2 alterations are mainly present in the CIN subtype. These aberrations mainly consist of missense mutations (74%) and, only to a lesser extent, insertions (22%) or fusions (0.7%). HER2 expression is more common in the intestinal subtype according to Lauren’s classification, as well as proximal gastric cancer or GEJ cancer, metastatic disease (especially liver metastasis), and lymph node invasion.
HER2+ Gastric Cancer (including GEJ) Diagnosis
A diagnosis of HER2+ gastric cancer is made by testing a biopsy sample from the stomach tumor using Immunohistochemistry (IHC) to assess the level of HER2 protein expression, with a positive result typically indicated by a score of 3+ on IHC; if the IHC score is equivocal (2+), further testing with Fluorescence In Situ Hybridization (FISH) is required to confirm gene amplification and definitively diagnose HER2 positivity.
Additionally, the following tests and procedures are used to diagnose gastric cancer
- Medical history and physical examination
- Laboratory testing
- Upper endoscopy with biopsy
- Barium swallow
- Computed Tomography (CT) scan
- Magnetic Resonance Imaging (MRI) with gadolinium
- Biomarker testing
- HER2
- PD-L1
- Microsatellite instability
- Mismatch repair deficiency
- Tumor mutational burden
- NTRK
Further details related to country-based variations are provided in the reported.
HER2+ Gastric Cancer (including GEJ) Treatment
The outcomes of standard treatment for gastric cancer are generally poor, except in cases of highly localized cancers. Newly diagnosed patients with gastric cancer represent potential candidates for studies investigating novel treatment approaches. Various treatment options are available for gastric cancer, with the primary focus being on different forms of standard therapies, including
- Endoscopic mucosal resection
- Surgery
- Gastrectomy
- Radiation therapy
- Chemotherapy
- Targeted therapy
- Ramucirumab
- Regorafenib
- Trastuzumab
- Trastuzumab deruxtecan
- Immunotherapy
- Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
Further details related to country-based variations are provided in the reported.
HER2+ Gastric Cancer (including GEJ) Epidemiology
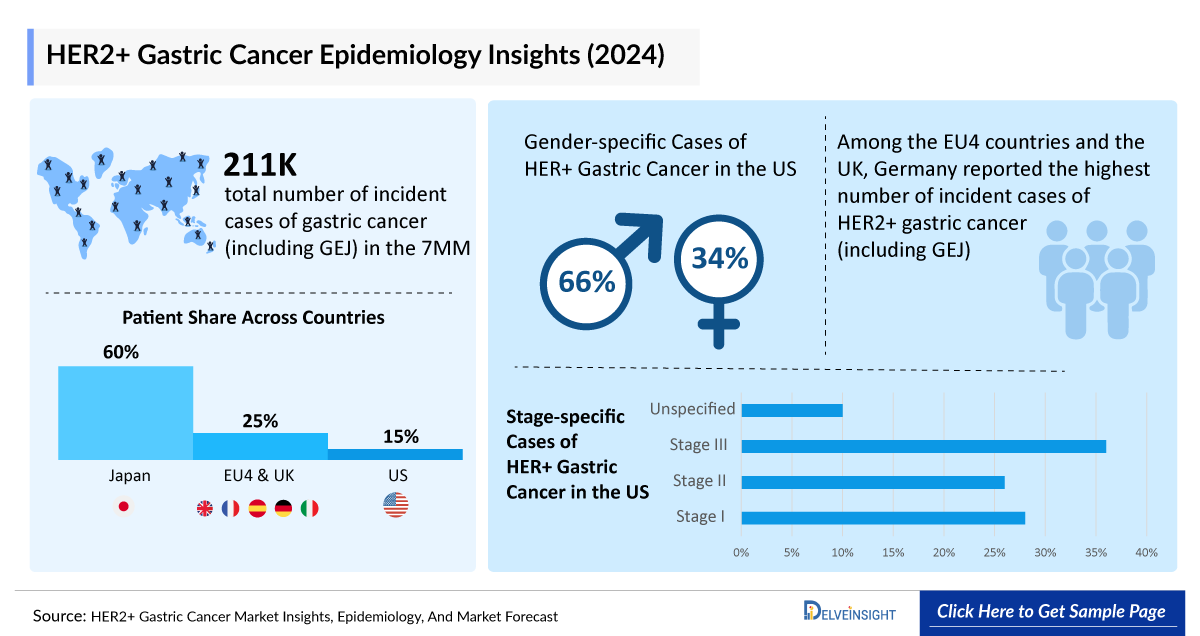
As the market is derived using a patient-based model, the HER2+ gastric cancer (including GEJ) epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total incident cases of gastric cancer (including GEJ), gender-specific cases of gastric cancer (including GEJ), stage-specific cases of gastric cancer (including GEJ), total incident cases of HER2+ gastric cancer (including GEJ), and total incident cases of advanced HER2+ gastric cancer (including GEJ), in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034. The total incident cases of gastric cancer (including GEJ) in the 7MM comprised approximately 211,560 cases in 2024 and are projected to increase during the forecasted period.
Key finding from the HER2+ Gastric Cancer Epidemiological Analysis and Forecasts:
- The total number of incident cases of gastric cancer (including GEJ) in the 7MM accounted for approximately 211,550 in 2024. These cases are expected to increase by 2034.
- In 2024, Japan contributed to the largest incident cases of HER2+ gastric cancer, acquiring ~60% of the 7MM cases. Whereas EU4 and the UK, accounted for around 25% of the total population share, respectively, in 2024.
- Among the EU4 countries and the UK, Germany reported the highest number of incident cases of HER2+ gastric cancer (including GEJ) in 2024, with approximately 3,400 cases, followed by Italy, with around 3,000 cases. In contrast, the UK recorded the fewest incident cases of HER2+ gastric cancer (including GEJ).
- In the US, among gender in gastric cancer (including GEJ), males accounted for the highest number of cases, i.e., 66% in 2024.
- In Japan, a significant portion of gastric cancer cases are diagnosed at an early stage, contributing to better overall survival rates, largely due to effective screening programs and early detection methods. In Japan, among the total diagnosed cases of gastric cancer (including GEJ) by stage, Stage I accounted for the highest number of cases, with approximately 95,400 in 2024, followed by Stage III with approximately 12,600 cases.
HER2+ Gastric Cancer Epidemiology Segmentation:
- Total incident cases of gastric cancer (including GEJ)
- Gender-specific cases of gastric cancer (including GEJ)
- Stage-specific cases of gastric cancer (including GEJ)
- Total incident cases of HER2+ gastric cancer (including GEJ)
- Total incident cases of advanced HER2+ gastric cancer
HER2+ Gastric Cancer (including GEJ) Drug Chapters Analysis
The drug chapter segment of the HER2+ gastric cancer (including GEJ) report encloses a detailed analysis of HER2+ gastric cancer (including GEJ) marketed drugs and late-stage (Phase III, Phase II/III, Phase II, Phase I/II, and Phase I) pipeline drugs. It also helps understand the HER2+ gastric cancer (including GEJ) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
HER2+ Gastric Cancer Marketed Drugs
KEYTRUDA (pembrolizumab): Merck
KEYTRUDA is an anti-PD-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. KEYTRUDA is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes, which may affect both tumor cells and healthy cells.
KEYTRUDA, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is also approved for the first-line treatment of adults with locally advanced unresectable or metastatic HER2– gastric or GEJ adenocarcinoma.
In November 2023, the FDA revised the existing indication of KEYTRUDA with trastuzumab, fluoropyrimidine, and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2+ gastric or GEJ adenocarcinoma. This updated indication, which remains approved under accelerated approval regulations, restricts its use to patients whose tumors express PD-L1 (Combined Positive Score {CPS =1}) as determined by an FDA-approved test.
In May 2024, the Ministry of Health, Labour, and Welfare (MHLW) approved KEYTRUDA for the treatment of unresectable advanced or recurrent gastric cancer.
ENHERTU (trastuzumab deruxtecan): Daiichi Sankyo and AstraZeneca
ENHERTU is a HER2-directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC technology, ENHERTU is the lead ADC in the oncology portfolio of Daiichi Sankyo and the most advanced program in AstraZeneca’s ADC scientific platform. ENHERTU is also approved for unresectable or metastatic HER2+ breast cancer, unresectable or metastatic HER2-low breast cancer, unresectable or metastatic NSCLC, locally advanced or metastatic HER2+ gastric or GEJ adenocarcinoma, and unresectable or metastatic HER2+ solid tumors.
In January 2021, the US FDA-approved ENHERTU for the treatment of adult patients with locally advanced or metastatic HER2+ gastric or GEJ adenocarcinoma who have received a prior trastuzumab-based regimen.
In December 2022, ENHERTU was approved in the European Union as a monotherapy for the treatment of adult patients with advanced HER2+ gastric or GEJ adenocarcinoma who have received a prior trastuzumab-based regimen.
HER2+ Gastric Cancer Emerging Drugs
ZIIHERA (zanidatamab): Jazz Pharmaceuticals, BeiGene, and Zymeworks
ZIIHERA is a bispecific HER2-directed antibody that binds to two extracellular sites on HER2. In November 2024, the FDA granted accelerated approval to ZIIHERA for adult patients with previously treated, unresectable, or metastatic HER2+ (immunohistochemistry 3+) Biliary Tract Cancer (BTC), as detected by an FDA-approved test.
Jazz Pharmaceuticals is looking forward to the potential further development of zanidatamab in the neoadjuvant/adjuvant GEA population. Furthermore, Jazz and BeiGene are developing Zanidatamab under license agreements from Zymeworks, which first developed the molecule.
Jazz Pharmaceuticals anticipates a top-line data readout in the second quarter of 2025 from the pivotal Phase III HERIZON-GEA-01 trial (NCT05152147), which is evaluating zanidatamab in combination with chemotherapy, with or without tislelizumab, as a first-line treatment for HER2-expressing metastatic GEA.
Additionally, according to Jazz Pharmaceuticals’ presentation published in January 2025, the company anticipates the path to approval in first-line GEA with anticipated sBLA submission in 2025.
HLX22 (AC101): Shanghai Henlius Biotech and AbClon
HLX22 is an innovative anti-HER2 monoclonal antibody introduced from AbClon and further investigated and developed by Henlius. Pre-clinical studies showed that the co-treatment with HLX22 and trastuzumab synergistically inhibited tumor cell proliferation and apoptosis, which led to enhanced antitumor activity in vitro and in vivo. A Phase I clinical trial of HLX22 demonstrated that HLX22 was well tolerated and had a favorable safety profile.
In October 2024, Henlius announced that the Clinical Trial Notification (CTN) for Phase III international multicenter clinical study of HLX22, in combination with trastuzumab and chemotherapy for the first-line treatment of HER2+ advanced gastric cancer, had been permitted by Japan’s PMDA.
In May 2024, Henlius Biotech announced that the US FDA had approved the IND for Phase III international multicenter clinical study of HLX22.
HER2+ Gastric Cancer Drug Class Analysis
HER2-directed Therapies
HER2-directed therapies have markedly advanced the treatment of HER2+ gastric cancer by specifically targeting the HER2 receptor, which is overexpressed in these tumors. Trastuzumab, a monoclonal antibody, binds to HER2, preventing its activation and inducing antibody-dependent cellular cytotoxicity. Additionally, therapies such as trastuzumab deruxtecan also target HER2, with the latter delivering a cytotoxic payload directly to cancer cells. These innovative treatments have demonstrated significant improvements in survival rates.
PD-1 Inhibitor
PD-1 inhibitors, such as pembrolizumab, play a crucial role in the treatment of HER2+ gastric cancer by boosting the immune response against cancer cells. When used in combination with trastuzumab and chemotherapy, pembrolizumab has demonstrated significant clinical benefits, including enhanced tumor response rates and the potential for improved survival in patients with HER2+ gastric or GEJ adenocarcinoma. The combination of PD-1 inhibitors with HER2-directed therapies offers a promising strategy to synergistically target both immune evasion mechanisms and HER2 signaling pathways in cancer cells.
HER2+ Gastric Cancer (including GEJ) Market Outlook
The treatment landscape for HER2+ gastric cancer has evolved significantly with the approval of HERCEPTIN in the first-line setting, followed by the integration of KEYTRUDA in combination with trastuzumab and chemotherapy, and the subsequent approval of ENHERTU in the second-line setting. These advancements have shifted the therapeutic paradigm from conventional chemotherapy-based approaches to more targeted and immune-based strategies, improving survival outcomes for patients with HER2+ gastric and GEJ adenocarcinoma. Earlier, se ond-line treatment options for HER2+ gastric cancer were limited, with chemotherapy (paclitaxel or irinotecan) remaining the standard of care after trastuzumab progression. However, the emergence of ENHERTU displaced chemotherapy as the preferred second-line therapy.
Various new therapies are in development with a focus on mentioned limitations of the currently approved drugs. Some of the potential ones include Zanidatamab (Jazz Pharmaceuticals), Rilvegostomig (AstraZeneca), Evorpacept (ALX Oncology), HLX22 (Shanghai Henlius Biotech), and others with their candidates in different stages of clinical development. The launch of these therapies in the future would bring a positive shift in the treatment landscape of HER2+ gastric cancer. The overall market is projected to grow, driven by increasing biomarker testing, better patient selection, and continued advancements in HER2-targeted ADCs, bispecific antibodies, and novel immune-based approaches.
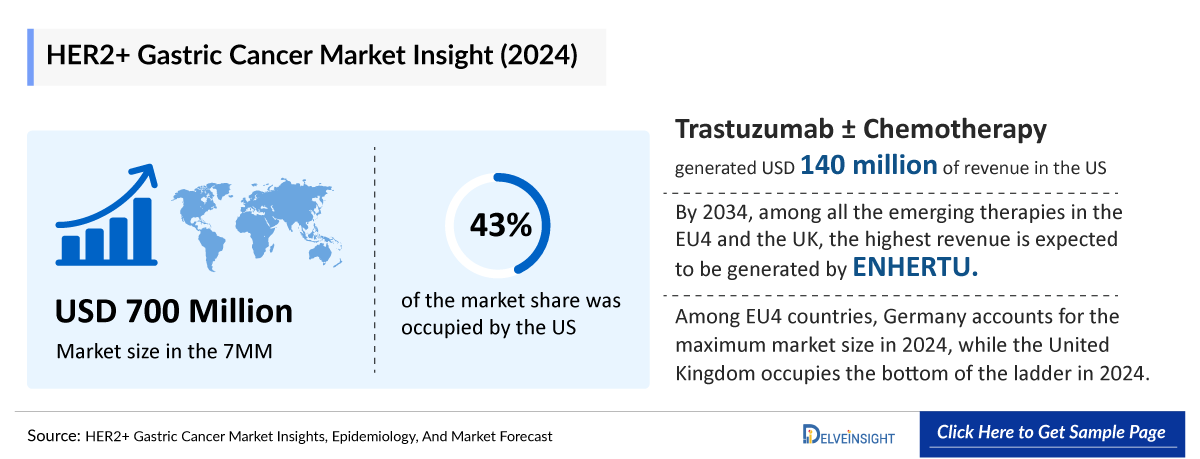
- The total market size of HER2+ gastric cancer (including GEJ) in the 7MM is approximately USD 700 million in 2024 and is projected to increase during the forecast period (2025–2034).
- Among EU4 countries, Germany accounts for the maximum market size in 2024, while the United Kingdom occupies the bottom of the ladder in 2024.
- In 2024, among the current therapies for HER2+ gastric cancer, the largest revenue was generated by Trastuzumab ± Chemotherapy, i.e., USD ~140 million in the United States.
- By 2034, among all the emerging therapies in the EU4 and the UK, the highest revenue is expected to be generated by ENHERTU.
Further details will be provided in the report….
HER2+ Gastric Cancer Market Recent Developments and Breakthroughs
- In the January 2025 presentation, Jazz Pharmaceuticals anticipates the potential approval and launch of zanidatamab as a first-line treatment for GEA in 2026. Additionally, the company is also looking forward to expanding its market strategy for zanidatamab in 2026.
- In February 2025, AstraZeneca anticipates a data readout from the Phase Ib/II (NCT04379596, DESTINY-Gastric03) trial, which is focused on treating metastatic or unresectable HER2+ gastric cancer, GEJ, and GEA, in 2026.
- In February 2025, AstraZeneca expects the first patient dosing of ENHERTU (NCT06731478, DESTINY-Gastric05) for HER2+ first-line locally advanced or metastatic gastric cancer or GEJ adenocarcinoma to begin in Q1 2025, with data anticipated to be available after 2026.
- AstraZeneca anticipates data from the DESTINY-Gastric04 (NCT04704934) trial, which is focused on treating HER2+ gastric cancer or GEJ adenocarcinoma patients who have progressed on or after a trastuzumab-containing regimen and have not received any additional systemic therapy, to be available in H2 2025.
- In January 2025, ALX Oncology presented positive updated data from the ASPEN-06 Phase II clinical trial at the 2025 ASCO Gastrointestinal Cancers Symposium, demonstrating that the company's investigational CD47-blocker, evorpacept, produces a durable clinical response and exhibits a well-tolerated safety profile in patients with previously treated HER2+ advanced gastric cancer or GEJ cancer.
HER2+ Gastric Cancer (including GEJ) Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034.
In front-line HER2-positive gastric cancer, HERCEPTIN (trastuzumab) is approved; however, it has been replaced over time by newer medications and is now administered as a first-line combination. Not only emerging competition but the introduction of HERCEPTIN’s biosimilar has led to price erosion and increased accessibility for treating HER2+ gastric cancer, with biosimilars demonstrating similar efficacy and safety profiles to the reference drug.
In addition, based on the Destiny-Gastric01 trial, ENHERTU was licensed for use in post-HERCEPTIN, HER2-positive gastric cancer; in this setting, ENHERTU demonstrated an ORR of 41%. The market is currently dominated by trastuzumab ± chemotherapy, however, by 2034 ENHERTU and zanidatamab are expected to take the lead.
Further detailed analysis of emerging therapies drug uptake in the report…
HER2+ Gastric Cancer (including GEJ) Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II/III, Phase II, Phase I/II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for HER2+ gastric cancer’s (including GEJ) emerging therapies.
Latest KOL Views on HER2+ Gastric Cancer
To keep up with current market trends, we take KOLs and SME's opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the HER2+ gastric cancer (including GEJ) evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, including MD, PhD, Instructor, and Postdoctoral Researcher, Professor, Researcher, and Others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 6+ KOLs in the 7MM. Centers such as Memorial Sloan Kettering Cancer Center, University of Texas MD Anderson Cancer Center, Memorial Sloan Kettering Cancer Center, National Cancer Center Hospital East in Kashiwa, Università Vita-Salute San Raffaele, San Raffaele Scientific Institute, St. Marianna University School of Medicine, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or HER2+ gastric cancer (including GEJ) market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on HER2+ Gastric Cancer Patient Trends?
- In United States, ‘HER2 status changed the treatment paradigm for gastric cancers starting in 2010 with the addition of the anti-HER2 monoclonal antibody trastuzumab as a treatment option used in addition to chemotherapy. More recently, the US FDA approved the PD-L1 inhibitor pembrolizumab in combination with trastuzumab, which has drastically improved responses and led to improvements in PFS in patients with HER2+ disease.’
- In Japan, ‘ETRP showed promising activity for patients with HER2+ gastric and GEJ cancer with a response rate above 41.3% compared with 26.6% with TRP alone. The benefit of evorpacept was especially observed in patients with confirmed HER2+ [expression] in fresh biopsy or ctDNA, in that its mode of action was able to enhance the activity of trastuzumab. This efficacy, as well as acceptable safety profile, support the further development of [evorpacept plus] TRP in patients with gastric and GEJ cancers.’
Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Scope of the HER2+ Gastric Cancer Report
- The report covers a segment of key events, an executive summary, descriptive overview of HER2+ gastric cancer (including GEJ), explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the HER2+ gastric cancer (including GEJ) market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM HER2+ gastric cancer (including GEJ) market.
- The report also provides 2025 ASCO Gastrointestinal Cancers Symposium insights on HER2+ gastric cancer.
HER2+ Gastric Cancer (including GEJ) Report Insights
- Patient population
- Therapeutic approaches
- HER2+ gastric cancer (including GEJ) pipeline analysis
- HER2+ gastric cancer (including GEJ) market size and trends
- Existing and future market opportunity
HER2+ Gastric Cancer (including GEJ) Report Key Strengths
- Ten years forecast
- 7MM coverage
- HER2+ gastric cancer (including GEJ) epidemiology segmentation
- Key cross competition
- Highly analyzed market
- Drugs uptake
HER2+ Gastric Cancer (including GEJ) Report Assessment
- Current treatment practices
- Unmet needs
- Pipeline product profiles
- Market attractiveness
- Qualitative analysis (SWOT and conjoint analysis)
FAQs
Market Insights
- What was the HER2+ gastric cancer total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors for this growth?
- At what CAGR, the HER2+ gastric cancer market is expected to grow at the 7MM level during the study period (2020–2034)?
- How is Japan's HER2+ gastric cancer competitive landscape evolving?
- How will upcoming emerging therapies are going to impact ENHERTU's market share?
- Which drug is going to dominate the HER2+ gastric cancer market size by 2034?
- Which class is going to be the largest contributor in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of HER2+ gastric cancer?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to HER2+ gastric cancer?
- What is the historical and forecasted HER2+ gastric cancer patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What factors are affecting the increase in the diagnosis of symptomatic cases?
- What factors drive the geographical variation of HER2+ status in gastric cancer?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of HER2+ gastric cancer? What are the current treatment guidelines for the treatment of HER2+ gastric cancer in the US and Europe?
- How many companies are developing therapies for the treatment of HER2+ gastric cancer?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of HER2+ gastric cancer?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What key designations have been granted for the emerging therapies for HER2+ gastric cancer?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What is the 7MM historical and forecasted market of HER2+ gastric cancer?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the HER2+ gastric cancer market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand KOLs’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.