Human Papillomavirus 16-positive Cancers Market
Key Highlights
- Despite the progress in the HPV disease landscape, there is no FDA-approved therapy to date and patients rely on prophylactic treatment options.
- Three vaccines are approved to protect people from the high-risk HPV strains that cause the most cancers. Two of these vaccines are approved for use in all males and females (Cervarix [Glaxo SmithKline] and Gardasil [Merck]) while one is approved only for females [Gardasil 9 (Merck)].
- Key players such as Nykode Therapeutics (VB10.16), ISA Pharmaceuticals (ISA101b), and others are involved in developing therapies for HPV16+ Cancers.
- The US Food and Drug Administration (FDA) has granted Fast Track designation to ISA Pharmaceuticals' lead product, ISA101b, for the treatment of recurrent and metastatic oropharyngeal cancer (OPC) that is positive for Human Papillomavirus type 16 (HPV16). Additionally, ISA101b has received Orphan Drug designation for the treatment of HPV16-positive cervical cancer.
- ISA Pharmaceuticals presented an oral abstract at the American Society of Clinical Oncology (ASCO) Annual Meeting, showcasing results from a randomized trial of its lead therapeutic vaccine, ISA101b, in advanced head and neck cancer. These results offered crucial insights into the clinical application of cancer vaccines.
- In April 2024, Nykode Therapeutics announced the initiation of the Phase II clinical trial VB-C-04. The trial evaluates VB10.16 for HPV16-positive cancers, alone or in combination with Roche’s checkpoint inhibitor atezolizumab (TECENTRIQ) in patients with HPV16-positive, PD-L1-positive, recurrent, or metastatic cervical cancer.
- Recently, Nykode Therapeutics has announced a strategic shift for VB10.16. The company will now concentrate0020on the development of VB10.16 on locally advanced cervical cancer and recurrent metastatic head and neck cancer. This decision follows a thorough review and feedback from key opinion leaders and potential partners, aimed at optimizing resources and development timing.
DelveInsight's “HPV16+ Cancer– Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of HPV16+ Cancer epidemiology, market, and clinical development in HPV16+ Cancer. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the HPV16+ Cancer market trends in the United States, EU4 (Germany, Spain, Italy, and France) the United Kingdom, and Japan.
The HPV16+ Cancer market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted HPV16+ Cancer market size from 2020 to 2034 in 7MM. The report also covers current HPV16+ Cancer treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
HPV16+ Cancer Epidemiology
|
Segmented by: Total Incident Cases of Selected Cancers (Cervical Cancer, Anal Cancer, Oropharyngeal Cancer, Vaginal Cancer, Vulvar Cancer, and Penile Cancer) Total Incident Cases of Selected Cancers by HPV Status Total Incident Cases of HPV16+ Cancers Stage-specific Cases of HPV16+ Cancers |
|
HPV16+ Cancer key companies |
Nykode Therapeutics ISA pharmaceuticals |
|
HPV16+ Cancer key therapies |
VB10.16 ISA101b |
|
HPV16+ Cancer Market |
Segmented by: · Region · Therapies |
|
Analysis |
· KOL Views · SWOT Analysis · Reimbursement · Conjoint Analysis · Unmet needs |
HPV16+ Cancer Understanding and Treatment Algorithm
HPV16+ Cancer Overview and Diagnosis
Human papillomavirus (HPV) is a double-stranded DNA virus that infects squamous cells such as skin, oral, vaginal, anal, and nasal epithelium. The basal cells in the epithelium are the targets and, once infected, act as the reservoir for the virus. Viral replication occurs mainly in the epithelial cells that have completed cell division and are progressing to desquamation. Desquamation releases the viral particles and completes the viral replication cycle. There are over 130 different subtypes of HPV. Some infect mucosal epithelium, and some infect cutaneous epithelium, some subtypes have high oncogenic potential while others do not. Worldwide, the major oncogenic HPV subtypes are 16 and 18, which account for most of the HPV-related cancers. Diagnosis of HPV16+ cancer typically begins with screening tests that detect the presence of high-risk HPV DNA in cervical cells, such as the HPV test or the combined HPV/Pap co-test. If high-risk HPV, particularly HPV16, is detected, further evaluation through colposcopy and biopsy is often warranted to assess for dysplasia or malignant transformation. Although FDA-approved tests for HPV-related cancers are currently limited to cervical cancer, ongoing research aims to develop reliable diagnostic tools for detecting HPV16+ pre-cancers and cancers in other anatomical sites.
The HPV16+ Cancer report provides an overview of HPV16+ Cancer pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report.
HPV16+ Cancer Treatment
Treatment of HPV16+ cancer primarily involves the ablation of lesions through methods such as surgical excision, cryotherapy, electrocautery, and laser therapy. These approaches are aimed at removing or destroying cancerous tissues caused by persistent HPV16 infection. Surgical excision is often preferred for extensive lesions, while cryotherapy and electrocautery are used for smaller, resistant lesions, offering precise and immediate tissue destruction. Laser therapy provides control over the depth and spread of destruction, particularly useful in sensitive areas like the vagina. Post-procedure care typically includes the application of silver sulfadiazine cream to prevent infection and manage pain. While these treatments focus on managing the cancer, preventive HPV vaccines remain crucial in reducing the incidence of HPV16-related malignancies.
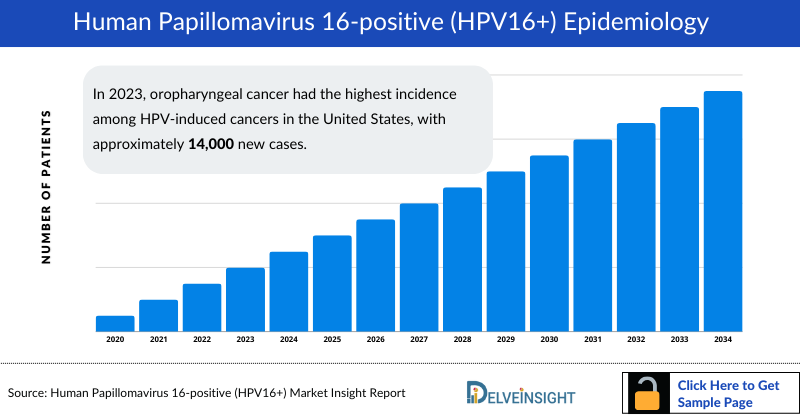
HPV16+ Cancer Epidemiology
The HPV16+ Cancer epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total Incident Cases of Selected Cancers (Cervical Cancer, Anal Cancer, Oropharyngeal Cancer, Vaginal Cancer, Vulvar Cancer, and Penile Cancer), total Incident Cases of Selected Cancers by HPV Status, total Incident Cases of HPV16+ Cancers, stage-specific Cases of HPV16+ Cancers in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- In 2023, oropharyngeal cancer had the highest incidence among HPV-induced cancers in the United States, with approximately 14,000 new cases.
- In 2023, the United States reported the highest number of incident cases of HPV-induced cancers among 7MM, with approximately 43,000 cases.
- In the United States, the total incident cases of HPV16+ cancer were nearly 35,000 cases in 2023.
- Among the EU4 and the UK, Germany had the highest incidence of HPV-induced cancers in 2023, followed by France. Conversely, Spain reported the lowest number of incident cases that year.
- In 2023, localized cases made up the highest number of incident cases of HPV-induced cancers.
HPV16+ Cancer Drug Chapters
The drug chapter segment of the HPV16+ Cancer report encloses a detailed analysis of HPV16+ Cancer-marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the HPV16+ Cancer pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Drug
Currently, there are no approved therapeutic vaccines specifically for treating HPV-induced cancers. The available vaccines, such as Gardasil and Cervarix, are prophylactic, meaning they are designed to prevent HPV infections and the associated cancers rather than treat existing conditions. These vaccines effectively reduce the incidence of cervical and other HPV-related cancers by preventing infection with high-risk HPV types. Although there is ongoing research into therapeutic vaccines aimed at eliciting immune responses against existing HPV infections and lesions, none have yet received approval for clinical use. Efforts continue to develop therapeutic options that could complement existing prophylactic vaccines and improve treatment outcomes for individuals with HPV-related malignancies.
Note: Detailed current therapy assessment will be provided in the full report of HPV16+ Cancer.
Emerging Drugs
VB10.16: Nykode Therapeutics/Roche
VB10.16 is a potentially first-in-class off-the-shelf therapeutic DNA-based cancer vaccine candidate in development for the treatment of human papillomavirus type 16 (HPV16)-positive cancers. The cancer vaccine is designed based on Nykode’s Vaccibody technology platform of targeting antigens to antigen-presenting cells. VB10.16 has reported promising data from a Phase II trial in advanced PD-L1 positive cervical cancer patients (NCT04405349) in combination with atezolizumab with mOS not reached, but at least 24 months at the time of analysis. The vaccine-induced significant HPV16-specific T-cell responses were correlated with clinical responses. The candidate has also demonstrated favorable clinical data in a Phase I/IIa study in pre-cancerous HPV16-induced high-grade cervical intraepithelial neoplasia demonstrating a statistically significant correlation of immune responses and clinical responses. Nykode is currently investigating VB10.16 in VB-C-03, an open-label, dose-finding Phase I/IIa trial evaluating VB10.16 in combination with MSD’s PD-1 inhibitor KEYTRUDA (pembrolizumab) in patients with HPV16-positive, PD-L1-positive, recurrent, or metastatic head and neck squamous cell carcinoma (HNSCC).
ISA101b: ISA Pharmaceuticals/Regeneron
ISA101b is a synthetic long peptide (SLP) therapeutic designed to mount a highly specific, broad, and durable, T-cell–mediated attack by the immune system on tumors positive for HPV16. It is being developed in collaboration with Regeneron Pharmaceuticals. The candidate is being evaluated in patients with HPV16+ oropharyngeal cancer, cervical cancer, and head and neck cancers. ISA101 has completed a Phase II trial in vulvar intra-epithelial neoplasia, establishing clinical proof-of-concept. In cervical cancer, ISA101 has completed a company-sponsored Phase I/II trial and has entered into further clinical development in collaboration with Regeneron. The alliance aims to develop and advance ISA101 in combination with cemiplimab (LIBTAYO), a PD-1 antibody currently under review by EMA and initially approved by the US FDA in September 2018 under the brand name LIBTAYO as monotherapy for patients with advanced cutaneous squamous cell carcinoma.
|
Table 1: Comparison of Key Emerging Drugs | ||||
|
Drug name |
Company |
MoA |
RoA |
Phase |
|
VB10.16 |
Nykode Therapeutics/Roche |
Antigen-presenting cell modulators |
Intramuscular |
II |
|
ISA101b |
ISA Pharmaceuticals/Regeneron |
Immunostimulant |
Subcutaneous |
II |
Note: Detailed emerging therapies assessment will be provided in the final report.
HPV16+ Cancer Market Outlook
The market outlook for HPV16+ cancer is promising, with significant advancements in emerging therapies. Current prophylactic vaccines, like Gardasil and Cervarix, prevent HPV infections but do not treat existing cancers. However, new therapeutic candidates such as Nykode Therapeutics' VB10.16, showing promise in combination with PD-1 inhibitors for cervical and head and neck cancers, and ISA Pharmaceuticals' ISA101b, which is advancing in trials for HPV16+ cancers, are generating optimism. These developments suggest potential improvements in treatment outcomes and market growth.
Key players, such as Nykode Therapeutics and ISA Pharmaceuticals are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of HPV16+ Cancer.
- The dynamics of the HPV16+ Cancer market are anticipated to change in the coming years owing to the expected launch of emerging therapies. The rising research, incidence, and screening, along with the emergence of novel therapies, will fuel the market during the forecast period of 2024–2034.
- Among 7MM, the United States accounts for the highest market size of HPV16+ Cancer in 2023.
- Among the EU4 and the UK, Germany had the highest market size in 2023, while Spain had the lowest market size for HPV16+ Cancer.
HPV16+ Cancer Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
HPV16+ Cancer Activities
The report provides insights into different therapeutic candidates in Phase II and Phase I stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for HPV16+ Cancer emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Doctors, Professors, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the Children's Hospital of Philadelphia (CHOP) Research Institute, Yale School of Medicine, the Walton Centre NHS Foundation Trust, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or HPV16+ Cancer market trends.
|
KOL Views |
|
“HPV is a significant public health threat, causing nearly 10% of all human cancers, including all cervical cancers and a high percentage of anal and oropharyngeal cancers.” |
|
“Compromised immune response and increased exposure to HPV heighten the risk of developing cervical cancer. Factors such as early sexual activity and multiple partners increase HPV exposure, which can lead to cervical cancer.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of HPV16+ Cancer, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the HPV16+ Cancer market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM HPV16+ Cancer market.
HPV16+ Cancer Report Insights
- Patient Population
- Therapeutic Approaches
- HPV16+ Cancer Pipeline Analysis
- HPV16+ Cancer Market Size and Trends
- Existing and future Market Opportunity
HPV16+ Cancer Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- HPV16+ Cancer Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
HPV16+ Cancer Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the historical and forecasted HPV16+ Cancer patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the HPV16+ Cancer total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which combination treatment approaches will have a significant impact on the HPV16+ Cancer drug treatment market size?
- What are the pricing variations among different geographies for approved therapy?
- What are the current and emerging options for the treatment of HPV16+ Cancer?
- How many companies are developing therapies for the treatment of HPV16+ Cancer?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the HPV16+ Cancer Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.



-cancers-pipeline.png&w=256&q=75)
