Hyperphosphatemia Market
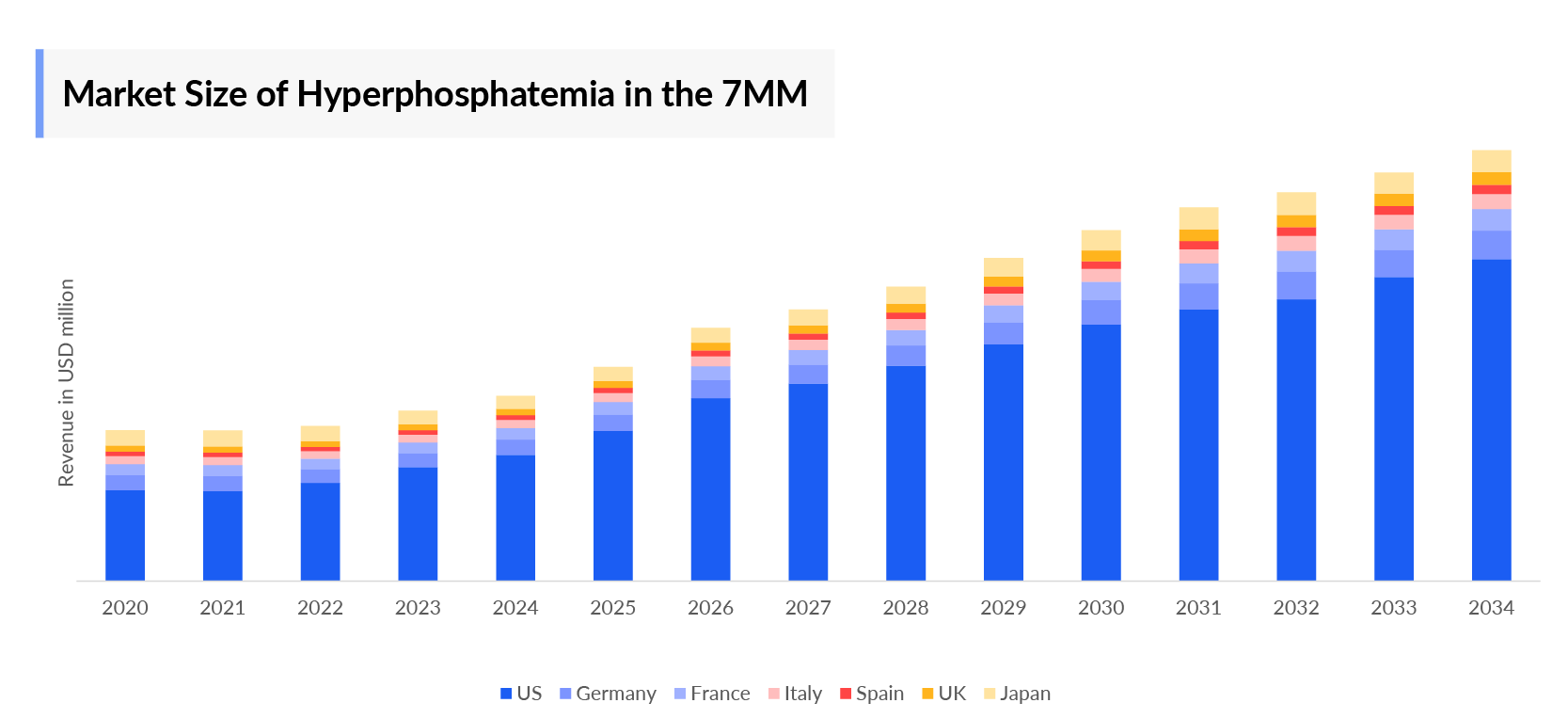
- The total hyperphosphatemia market size in the 7MM was ~USD 4,000 million in 2023. An increase in hyperphosphatemia market size is expected due to the rise in the diagnosed prevalence of CKD, thereby increasing the hyperphosphatemia patient pool.
- Hyperphosphatemia is a condition characterized by an increase in serum phosphate level above 4.5 mg/100 mL. The condition usually occurs due to a decrement in renal function, commonly among chronic kidney disease (CKD) patients, in which phosphate levels become abnormally high.
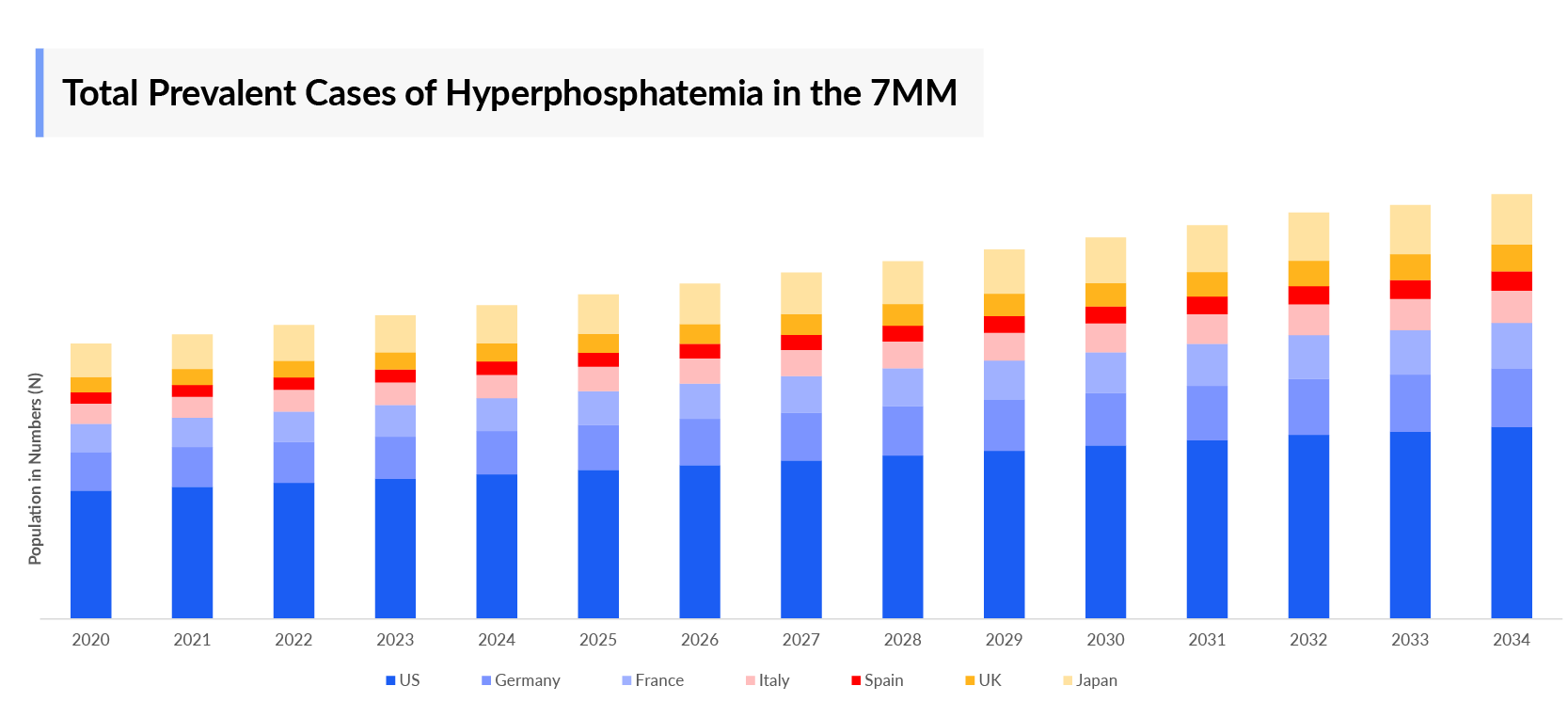
- In the 7MM, the United States had the highest prevalent cases, approximately 50% of the total prevalent pool in 2023.
- The market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of one-time gene therapies, and raised awareness.
- In 2023, the total number of prevalent cases of ESRD patients on dialysis in the United States was ~600,000, which as per DelveInsight’s estimates, is likely to increase in the forecasted period.
- FDA approved phosphate binder therapies for the treatment of the condition includes KIKLIN, AURYXIA/ RIONA and VELPHORO/ P-TOL.
- The United States accounts for the largest market size of Hyperphosphatemia, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- In October 2023, XPHOZAH received approval from the US FDA to control serum phosphate in adult chronic kidney disease patients who are on dialysis but can’t tolerate or have an inadequate response to treatment with a phosphate binder. In November 2023, the drug was granted Orphan Drug Designation for treating pediatric hyperphosphatemia.
- Oxylanthanum Carbonate (OLC), is emerging therapy offer promising potential for effectively treating the condition.
DelveInsight's “Hyperphosphatemia Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Hyperphosphatemia, historical and forecasted epidemiology as well as the Hyperphosphatemia market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Hyperphosphatemia market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Hyperphosphatemia market size from 2020 to 2034. The report also covers current Hyperphosphatemia treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Hyperphosphatemia Epidemiology |
Segmented by:
|
|
Hyperphosphatemia key companies |
|
|
Hyperphosphatemia key therapies/drug |
|
|
Hyperphosphatemia Market |
Segmented by:
|
|
Analysis |
|
Hyperphosphatemia Treatment Market
Hyperphosphatemia Overview, Country-Specific Treatment Guidelines and Diagnosis
Hyperphosphatemia typically lacks noticeable symptoms, often manifesting as uremic symptoms like fatigue, shortness of breath, and nausea. Elevated phosphate levels can lead to mineral and bone disorders, including calcification. It often arises from kidney disease but can also be linked to conditions like uncontrolled diabetes, hypoparathyroidism, or excessive phosphate intake.
The condition is diagnosed by serum phosphate measurement with specific blood tests that measure the levels of phosphate, calcium, magnesium, blood urea nitrogen, creatinine, Vitamin D and parathyroid hormone (PTH). It is important to diagnose and find the underlying cause of hyperphosphatemia to treat and restore normal phosphate metabolism.
Further details related to country-based variations in diagnosis are provided in the report
Hyperphosphatemia Treatment
Treatment for hyperphosphatemia depends on the underlying condition. For people with kidney disease, a combination of diet and medication is used to keep phosphate levels under control. In patients with good renal function, renal phosphate excretion can be increased through extracellular volume expansion by saline infusion and diuretics. If renal function is impaired, it is an indication for hemodialysis. Several treatment strategies that can be employed to tackle hyperphosphatemia include dietary restriction, phosphate binders (aluminum-based agents, calcium-based binders, magnesium carbonate, sevelamer, lanthanum carbonate, ferric citrate and sucroferric oxyhydroxide), drugs targeting intestinal phosphate transporters (tenapanor, nicotinic acid and nicotinamide), renal replacement therapies (peritoneal and hemodialysis) and management of secondary hyperparathyroidism.
Hyperphosphatemia Epidemiology
The Hyperphosphatemia epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Hyperphosphatemia epidemiology is segmented with detailed insights into Total Prevalent Cases of Chronic Kidney Disease (CKD), Total Diagnosed Prevalent Cases of Chronic Kidney (CKD), Stage-specific Distribution of Chronic Kidney (CKD), Total Prevalent Cases of End-Stage Renal Disease (ESRD), Number of ESRD patients undergoing Dialysis, Total Prevalent Cases of Hyperphosphatemia.
- Highest prevalent cases of Hyperphosphatemia in the 7MM was accounted by US ~500,000 cases in 2023.
- Among EU4 and the UK, Germany accounted for the highest prevalent cases ~56,000 cases in 2023.
- In 2023, Japan contributed second highest prevalent cases among 7MM ~300,000 cases.
- As per DelveInsight’s estimation, in 2023, the total number of prevalent cases of CKD stage 3–5 in the United States was ~2,400,000 cases.
Hyperphosphatemia Drug Chapter
The drug chapter segment of the Hyperphosphatemia market report encloses a detailed analysis of Hyperphosphatemia marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Hyperphosphatemia pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Hyperphosphatemia Drugs
VELPHORO/P-TAL: FRESENIUS MEDICAL CARE RENAL PHARMACEUTICALS
VELPHORO (polynuclear iron (III)–oxyhydroxide, sucroferric oxyhydroxide), developed by Vifor Pharma, received marketing authorization valid throughout Europe in August 2014, and the US FDA approved it in 2013. Marketed as P-TOL, in 2018, Kissei acquired exclusive development and marketing rights for P-TOL in Japan from Vifor Fresenius Medical Care Renal Pharma, and launched the drug in 2018 in Japan for the treatment of hyperphosphatemia. Launched in 30 countries, over 100,000 patients are now estimated to benefit from VELPHORO on a yearly basis.
KIKLIN: Astellas pharma
KIKLIN (bixalomer) launched by Astellas Pharma are amine-functional polymers which decrease the serum phosphorus concentration by binding to phosphate in the gastrointestinal tract, thus inhibiting absorption of phosphate into the human body. The drug was approved initially in 2012 for the treatment of hyperphosphatemia in patients on dialysis with chronic kidney disease and later the label was expanded in 2016 for the indication of treatment of hyperphosphatemia in patients with chronic kidney disease.
Note: Detailed current therapies assessment will be provided in the full report of Hyperphosphatemia
Emerging Hyperphosphatemia Drugs
Oxylanthanum Carbonate (OLC): Unicycive Therapeutics
Oxylanthanum carbonate (OLC) is an investigational phosphate binding agent developed by Unicycive Therapeutics for treating hyperphosphatemia in patients with chronic kidney disease (CKD). Utilizing proprietary nanoparticle technology, OLC aims to provide a more effective and patient-friendly alternative to existing treatments by reducing the pill burden, allowing patients to swallow smaller tablets rather than chew larger ones.
The drug has completed Phase II clinical trials, demonstrating favorable safety and tolerability profiles, and Unicycive plans to file a New Drug Application (NDA) with the FDA under the 505(b) (2) regulatory pathway. The global market for hyperphosphatemia treatments is projected to exceed $2.5 billion, highlighting the significant unmet need for effective therapies in this area.
Note: Detailed emerging therapies assessment will be provided in the final report.
Hyperphosphatemia Market Outlook
Key Hyperphosphatemia companies, such as Unicycive Therapeutics and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Hyperphosphatemia.
- US had the largest Hyperphosphatemia market size ~USD 2400 million in the 7MM.
- In 2023, Germany had the largest Hyperphosphatemia market size contribution among the EU4 and the UK with ~USD 150 million.
- The current Hyperphosphatemia market is primarily dominated by the use of phosphate binders (PBs) (calcium-based and calcium-free PBs) and other off-label medications.
Hyperphosphatemia Drugs Uptake
This section focuses on the uptake rate of potential Hyperphosphatemia drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Hyperphosphatemia companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Hyperphosphatemia Activities
The Hyperphosphatemia treatment market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The Hyperphosphatemia drugs market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Hyperphosphatemia emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging Hyperphosphatemia market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Jerry L Pettis VA Medical Center, University School of Medicine, Ambroise Pare´ University Hospital, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of Hyperphosphatemia. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
Region |
KOL Views |
|
United States |
"Even though less number of Velphoro pills need to be taken daily when compared to other phosphate binders, the cost of the drug is quite high, this is what I have heard from few physicians. However, more patients have started taking this drug as around 5-10% of dialysis patients are on Velphoro. Velphoro’s acceptance is still being questioned, however I am sure its efficacy is great, and we need more such therapies at a lower cost that can work wonders for this target pool. This market is in need of therapies that can increase the adherence on a daily basis along with lowering the phosphate levels to the desired value.” |
|
Germany |
"In several European countries such as Germany and the UK calcium-based PBs are preferred by a lot of physicians. However, the scenario is not the same in the United States where physicians are shifting more towards non-calcium based PBs. If we compare the pill burden among the countries, I think it is very difficult to analyze as it remains quite similar in Europe and the United States, both the countries have Velphoro launched, how good the drug is doing is important to understand.” |
|
Japan |
“See, around 8–12 PBs per day are prescribed to dialysis patients in Japan as majority of the advanced CKD patients do suffer from hyperphosphatemia and a major pill burden comprises of PBs. Sevelamer hydrochloride and Ca-containing binders are the majorly recommended PBs in Japan. I am not much aware about the scenario in the European countries but I know that there is a huge unmet need in china as well as in Taiwan, the physicians in these countries are prescribing too many medications an trust me they do not have any other option, looking for alternative is a task, which in turn lowers the compliance.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Hyperphosphatemia Market Access and Reimbursement
The Hyperphosphatemia market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Hyperphosphatemia Market Report
- The Hyperphosphatemia market report covers a segment of key events, an executive summary, descriptive overview of Hyperphosphatemia, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging Hyperphosphatemia therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Hyperphosphatemia market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Hyperphosphatemia market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Hyperphosphatemia market.
Hyperphosphatemia Market Report Insights
- Hyperphosphatemia Patient Population
- Therapeutic Approaches
- Hyperphosphatemia Pipeline Analysis
- Hyperphosphatemia Market Size
- Hyperphosphatemia Market Trends
- Existing and future Hyperphosphatemia Market Opportunity
Hyperphosphatemia Market Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Hyperphosphatemia Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging Hyperphosphatemia therapies
- Key Cross Competition
- Hyperphosphatemia Conjoint analysis
- Hyperphosphatemia Drugs Uptake
- Key Hyperphosphatemia Market Forecast Assumptions
Hyperphosphatemia Market Report Assessment
- Current Hyperphosphatemia Treatment Practices
- Hyperphosphatemia Unmet Needs
- Hyperphosphatemia Pipeline Product Profiles
- Hyperphosphatemia Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM Hyperphosphatemia treatment market?
- What was the Hyperphosphatemia market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the Hyperphosphatemia market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Hyperphosphatemia?
- How many Hyperphosphatemia companies are developing therapies for the treatment of Hyperphosphatemia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Hyperphosphatemia therapies?
Reasons to buy
- The Hyperphosphatemia market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Hyperphosphatemia treatment Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Hyperphosphatemia market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Hyperphosphatemia companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Hyperphosphatemia companies can strengthen their development and launch strategy.




.png&w=256&q=75)

