Hypertrophic Cardiomyopathy Market
- In 2023, the Hypertrophic Cardiomyopathy Market Size was highest in the US among the 7MM, accounting for approximately ~USD 506 Million, which is further expected to increase by 2034 at a CAGR of 21%.
- The Hypertrophic Cardiomyopathy Therapeutics Market is driven by increased awareness, advancements in genetic testing, and improved diagnosis, which drive demand for targeted therapies. The growing Hypertrophic Cardiomyopathy Prevalence, along with new treatments and increased R&D investments, further fuels market growth.
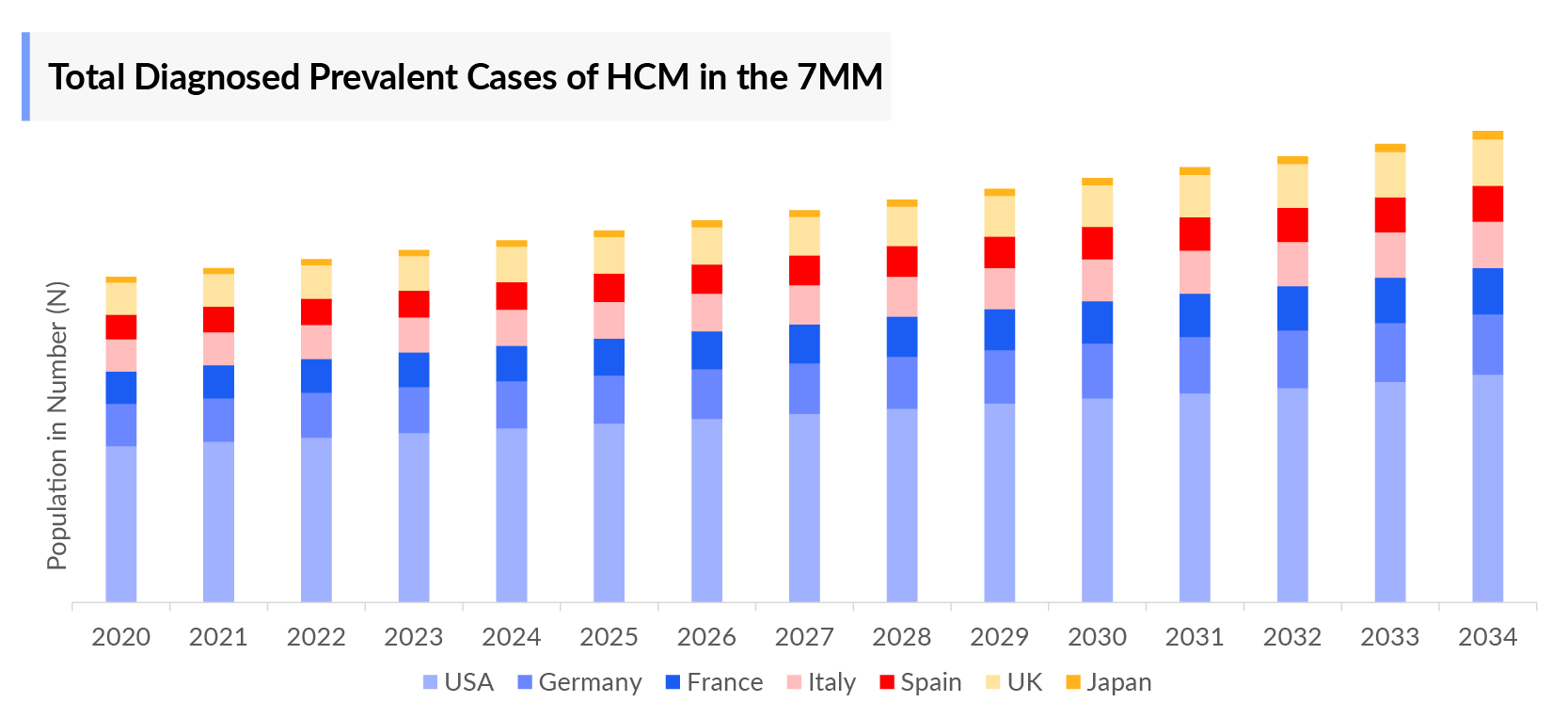
- According to DelveInsight’s estimates, the Hypertrophic Cardiomyopathy Diagnosed Prevalent Cases in the 7MM were ~246 thousand in 2023.
- The current Hypertrophic Cardiomyopathy Treatment includes CAMZYOS (mavacamten), beta-blockers, calcium antagonists, ACEs, ARBs, AADs, antiplatelet drugs, anticoagulant drugs and others, as approved therapies for the treatment of Hypertrophic Cardiomyopathy.
- In June 2023, European Commission approved CAMZYOS (mavacamten), for the Treatment of Symptomatic Obstructive Hypertrophic Cardiomyopathy. Earlier it received Positive CHMP opinion recommending approval in April 2023 based on positive Phase III EXPLORER-hypertrophic cardiomyopathy and VALOR-hypertrophic cardiomyopathy trials demonstrating benefit in patients receiving CAMZYOS versus placebo.
- In June 2024, Cytokinetics, Incorporated updated about initiation of clinical trial in Japan, the first participants have been dosed in a Phase I study evaluating the pharmacokinetics, safety and tolerability of aficamten in healthy Japanese and Caucasian participants.
Request for Unlocking the Sample Page of the "Hypertrophic Cardiomyopathy Treatment Market"
DelveInsight’s “Hypertrophic Cardiomyopathy Treatment Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Hypertrophic Cardiomyopathy, historical and forecasted epidemiology as well as the Hypertrophic Cardiomyopathy market trends in the United States, EU4 (Germany, France, Italy, Spain), the United Kingdom, and Japan.
The Hypertrophic Cardiomyopathy Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Hypertrophic Cardiomyopathy Market Size from 2020 to 2034. The Report also covers current Hypertrophic Cardiomyopathy Treatment practices, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Hypertrophic Cardiomyopathy Market |
|
|
Hypertrophic Cardiomyopathy Market Size |
~USD 506 Million in 2023 |
|
Hypertrophic Cardiomyopathy Companies |
La Jolla Pharmaceuticals, Par Pharmaceutical, Ono Pharmaceutical, Vivacelle Bio, Inotrem, Enlivex Therapeutics, Adrenomed, Shionogi, Asahi Kasei Pharma Corp., AM-Pharma, ABIONYX Pharma, Revimmune, Baxter Healthcare Corporation, BioMarck Pharmaceuticals, and others. |
Hypertrophic Cardiomyopathy Treatment Market
Hypertrophic cardiomyopathy is a heterogeneous myocardial disease, most often caused by autosomal dominant sarcomeric gene mutations, and represents the most common monogenic cardiomyopathy in humans. The disease is genetic heart condition characterized by marked variability in morphological expression and natural history, ranging from asymptomatic to heart failure or sudden cardiac death. Left ventricular hypertrophy and abnormal ventricular configuration result in dynamic left ventricular outflow obstruction in most patients.
Hypertrophic Cardiomyopathy Diagnosis
Diagnosing hypertrophic cardiomyopathy involves several steps. It begins with a thorough medical history and physical exam to identify symptoms like chest pain and family history of heart disease. The primary diagnostic tool is echocardiography, which images the heart to show thickening of the ventricular walls. An electrocardiogram (ECG) records electrical activity to reveal abnormalities associated with hypertrophic cardiomyopathy. Cardiac magnetic resonance imaging (MRI) may provide additional details if needed. Genetic testing can confirm the diagnosis, especially in cases with a strong family history. Additional tests like exercise testing or Holter monitoring may be used for further evaluation. Accurate diagnosis is crucial for effective management and treatment.
Further details related to diagnosis are provided in the report…
Hypertrophic Cardiomyopathy Treatment
Treatment for hypertrophic cardiomyopathy (hypertrophic cardiomyopathy) focuses on relieving symptoms, preventing complications, and improving quality of life. It typically involves medications such as beta-blockers and calcium channel blockers to reduce heart rate and manage symptoms, along with antiarrhythmic drugs for irregular heartbeats. Lifestyle modifications, including avoiding intense physical exertion and maintaining a healthy weight, are also important.
Severe cases may require interventional procedures like septal myectomy or alcohol septal ablation to address obstruction. Additionally, implantable devices like pacemakers or cardioverter-defibrillators (ICDs) may be used to manage arrhythmias and reduce the risk of sudden cardiac death. Regular follow-up with a cardiologist is crucial for ongoing monitoring and treatment adjustment.
Further details related to treatment are provided in the report…
Hypertrophic Cardiomyopathy Epidemiology
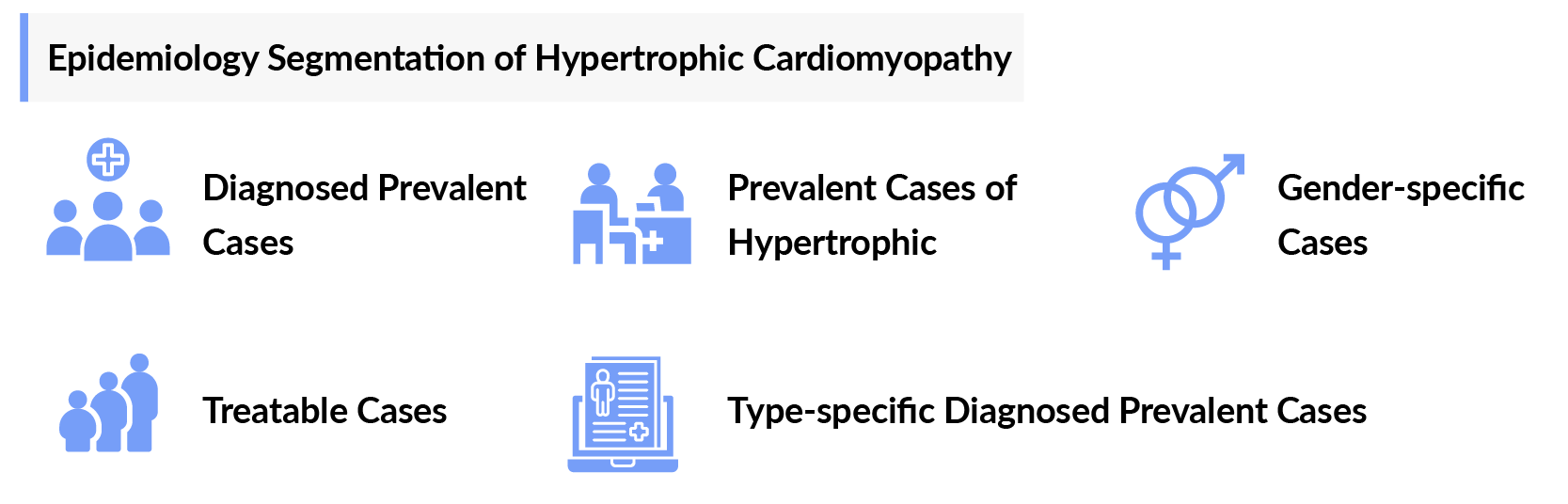
As the market is derived using a patient-based model, the Hypertrophic Cardiomyopathy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Prevalent Cases of Hypertrophic Cardiomyopathy, Diagnosed Prevalent Cases of Hypertrophic Cardiomyopathy, Gender-specific Diagnosed Prevalent Cases of Hypertrophic Cardiomyopathy, and Type-specific Diagnosed Prevalent Cases of Hypertrophic Cardiomyopathy, in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034.
- In the assessment done by DelveInsight, the estimated total Hypertrophic Cardiomyopathy Prevalent Cases in the 7MM were ~1,079 thousand in 2023.
- The highest tHypertrophic Cardiomyopathy diagnosed prevalent cases were accounted for by the US in 2023 (~118 thousand), which is expected to show a rise in the future.
- Among the European countries, Germany had the highest Hypertrophic Cardiomyopathy Diagnosed Prevalent Cases with ~32 thousand cases, followed by France, which had diagnosed prevalent population of ~25 thousand in 2023. On the other hand, Spain had the lowest diagnosed prevalent population (~19 thousand cases).
- Japan had ~4 thousand total diagnosed prevalent cases of Hypertrophic Cardiomyopathy in 2023, accounting for approximately 2% in 7MM.
- In 2023, in the US, the gender-specific diagnosed prevalent cases of Hypertrophic Cardiomyopathy were highest for males (~57%), as compared to females (~43%), which is attributed to factors such as hormonal differences and physiology, lifestyle and occupational factors and others.
- In the UK, among type-specific cases of hypertrophic cardiomyopathy in 2023, the highest number of cases was of Obstructive hypertrophic cardiomyopathy, with ~16,000 cases, while Non-obstructive hypertrophic cardiomyopathy had the least, with ~8,000 cases.
Hypertrophic Cardiomyopathy Drugs Market Chapters
The drug chapter segment of the Hypertrophic Cardiomyopathy treatment market report encloses a detailed analysis of Hypertrophic Cardiomyopathy off-label drugs and late-stage (Phase-III and Phase-II) Hypertrophic Cardiomyopathy pipeline drugs. It also helps to understand the Hypertrophic Cardiomyopathy clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Marketed Hypertrophic Cardiomyopathy Drugs
- Mavacamten: Bristol Myers Squibb
Mavacamten is an investigational, novel, oral, allosteric modulator of cardiac myosin; mavacamten reduces cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation, resulting in hypercontractility, left ventricular hypertrophy, and reduced compliance.
In Hypertrophic Cardiomyopathy Clinical and preclinical studies, mavacamten has consistently reduced biomarkers of cardiac wall stress, lessened excessive cardiac contractility, and increased diastolic compliance. MyoKardia is developing mavacamten to treat conditions in which excessive cardiac contractility and impaired diastolic filling of the heart are the underlying cause. Mavacamten is initially being developed for the treatment of symptomatic, obstructive hypertrophic cardiomyopathy (hypertrophic cardiomyopathy). Based on its mechanism of action and evidence of therapeutic activity, mavacamten is also being studied in the clinic to treat symptomatic non-obstructive hypertrophic cardiomyopathy and among a targeted population of patients with heart failure with preserved ejection fraction (HFpEF).
In April 2022, the US FDA first approved CAMZYOS (mavacamten) for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive hypertrophic cardiomyopathy (hypertrophic cardiomyopathy).
Currently, the company is developing CAMZYOS, which is in Phase III for Non-obstructive hypertrophic cardiomyopathy.
Emerging Hypertrophic Cardiomyopathy Drugs
- Aficamten (CK-274): Cytokinetics
Aficamten, or CK-274, is an investigational, novel, oral, small molecule cardiac myosin inhibitor discovered by company scientists independent of its collaborations for the potential treatment of hypertrophic cardiomyopathies (hypertrophic cardiomyopathy).
CK-274 was designed to reduce the hypercontractility associated with hypertrophic cardiomyopathy. Hypertrophic cardiomyopathy causes the heart to thicken and stiffen, eventually limiting its ability to pump blood. CK-274 addresses this hypercontractility by blocking some myosins from pulling, resulting in less contraction or fewer hands on the rope. In preclinical models, CK-274 reversed and reduced thickening and stiffening of the heart.
In December 2021, US FDA granted Breakthrough Therapy Designation for aficamten for the treatment of symptomatic obstructive hypertrophic cardiomyopathy (oHypertrophic cardiomyopathy). Aficamten is a next-generation cardiac myosin inhibitor in development for the potential treatment of hypertrophic cardiomyopathy.
Currently, the company is preparing for registration of CK-274 in second half of 2024 for the treatment of Obstructive Hypertrophic Cardiomyopathy.
- MYK-224: Bristol Myers Squibb
MYK-224 is a small molecule drug that is being developed by MyoKardia, a subsidiary of Bristol-Myers Squibb, to treat diastolic heart failure (HFpEF) and obstructive hypertrophic cardiomyopathy. It is taken orally and targets the heart's cardiac myosin motor protein to normalize filling and contractility.
Currently drug is in Phase II trial for treatment of Symptomatic Obstructive Hypertrophic Cardiomyopathy and MERCUTIO study is expected to completed by August 2024.
Further detail in the report…
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Aficamten |
Cardiac myosin inhibitors |
Oral |
Cytokinetics |
III |
|
MYK-224 |
Cardiac myosin modulators |
XX |
Bristol-Myers Squibb Company |
II |
|
XXX |
XXX |
XXX |
XXX |
II |
Hypertrophic Cardiomyopathy Market Outlook
Cardiomyopathy is a term that refers to abnormalities of heart muscle contractility, covering a heterogeneous range of etiologies. Hypertrophic cardiomyopathy is characterized by left ventricular hypertrophy and hypercontractility. It is almost always caused by mutations of genes encoding sarcomeric proteins.
Current pharmacological treatment strategies of Hypertrophic cardiomyopathy are mainly centered on managing the symptoms and minimizing disease progression. However, it is important to keep in mind that these strategies are not disease-specific since they target the neurohormonal system and excitation–contraction coupling (ECC) while the basic disease mechanism remains untreated. Nevertheless, pharmacological therapy plays an essential role in restoring quality of life (QoL) and reducing the risk of disease-related complications. The main goals of pharmacological therapy in Hypertrophic cardiomyopathy include control of symptoms and exercise limitation, abolition or reduction of dynamic intra-ventricular gradients, treatment of LV dysfunction and heart failure (HF), control of atrial fibrillation (AF) and ventricular arrhythmias, and prevention of cardioembolism. The current market forecast provided in the report solely focuses on the revenue generated by symptomatic therapies divided into their constituent therapeutic classes without any individual product-based market share due to the strong presence of a multitude of generic products for Hypertrophic cardiomyopathy management.
The primary revenue-generating components of the acute treatment market can be broadly classified into the following therapeutic classes: Beta-blockers, calcium antagonists, ACEI/ARBs, anti-arrhythmic drugs (AADs), antiplatelet drug, and anticoagulant drugs. Beta-blockers are the most popular and effective agents employed, generating significant revenue. Presently, atenolol, nadolol, bisoprolol, and metoprolol are some frequently used beta-blockers. While calcium antagonists are considered in patients who are intolerant or have contraindications to beta-blockers, angiotensin-converting enzyme inhibitors (ACEi), or angiotensin receptor blockers (ARBs), mainly target those patient segments suffering from concomitant systemic hypertension.
In the upcoming Hypertrophic Cardiomyopathy Treatment Market Landscape, there are a plethora of companies investigating agents for use in Hypertrophic Cardiomyopathy which includes Bristol Myers Squibb, Cytokinetics, and others. There are many more pharma companies which are conducting clinical trials for therapies for Hypertrophic Cardiomyopathy.
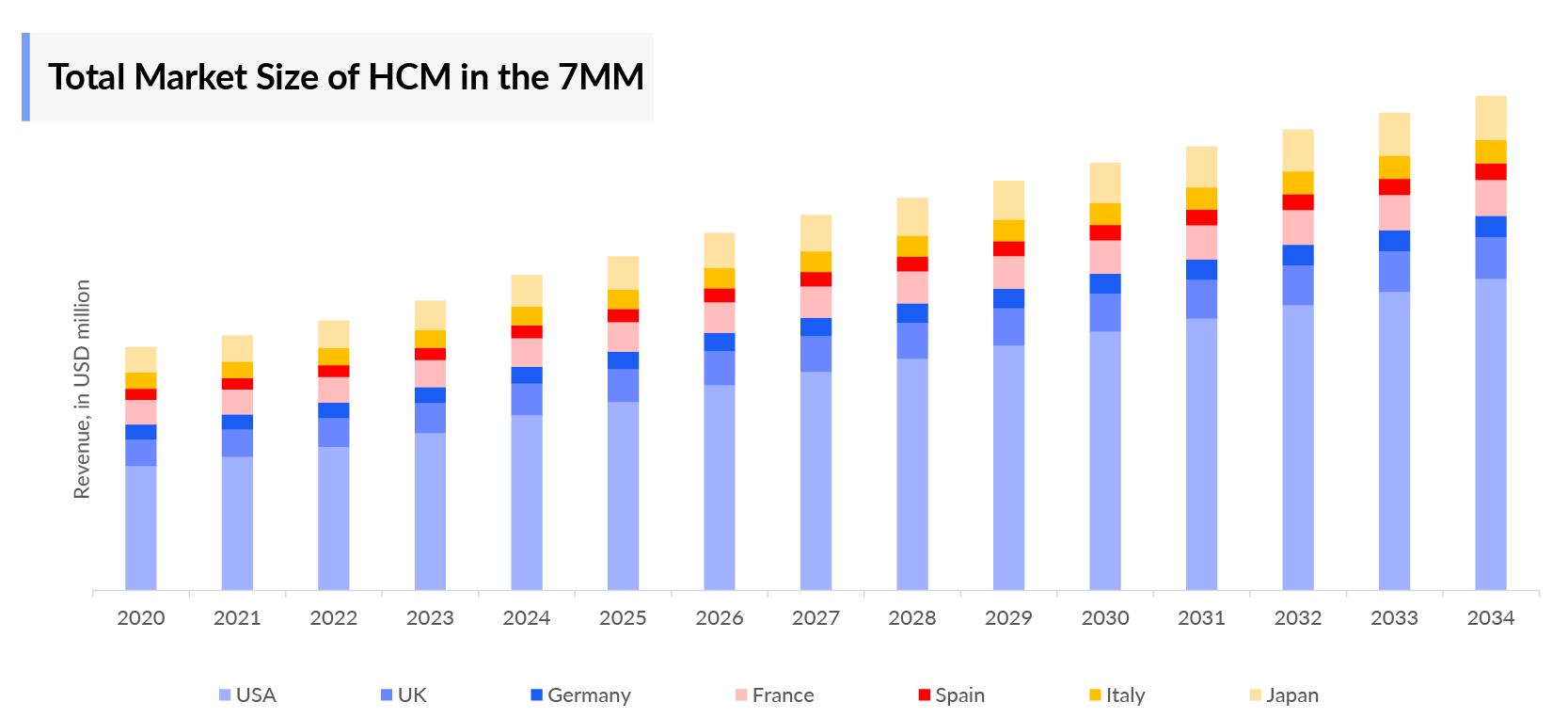
- The Hypertrophic Cardiomyopathy Therapeutics Market Size in the 7MM was approximately USD 646 million in 2023, The Hypertrophic Cardiomyopathy Treatment Market is expected to grow at a Compound annual growth rate (CAGR) of 20% during the forecast period (2024–2034) attributed to the new emerging therapies, and others.
- The United States accounted for the highest Hypertrophic Cardiomyopathy Market Size approximately 78% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the European countries, Germany had the highest Hypertrophic Cardiomyopathy market size with nearly USD 33 million in 2023, while Spain had the lowest Hypertrophic Cardiomyopathy market size with nearly USD 23 million in 2023.
- The Hypertrophic Cardiomyopathy Treatment Market Size in Japan was estimated to be nearly USD 5 million in 2023, which accounts for 1% of the total 7MM market.
- With the expected launch of upcoming Hypertrophic Cardiomyopathy therapies, such as Aficamten, the Hypertrophic Cardiomyopathy Market Size is expected to show a change in the upcoming years.
Hypertrophic Cardiomyopathy Drugs Uptake
This section focuses on the uptake rate of potential Hypertrophic Cardiomyopathy drugs expected to launch in the market during 2020–2034. For example, Aficamten in the US is expected to be launched by 2027 with a peak share of 10%. Aficamten is anticipated to take 5 years to peak with a medium uptake.
Hypertrophic Cardiomyopathy Pipeline Development Activities
The Hypertrophic Cardiomyopathy therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Hypertrophic Cardiomyopathy companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Hypertrophic Cardiomyopathy Therapeutics Market Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Hypertrophic Cardiomyopathy emerging therapies.
KOL Views
To keep up with current Hypertrophic Cardiomyopathy market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Hypertrophic Cardiomyopathy evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from University of Kansas, US; hypertrophic cardiomyopathy Institute, Tufts Medical Center, Boston, Massachusetts, US; Department of Otorhinolaryngology–Head and Neck Surgery, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, US; University of Siena, Italy; University of Southampton, Southampton, UK; University of Fukui, Fukui, Japan; Nippon Medical School, Tokyo, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Hypertrophic Cardiomyopathy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Hypertrophic Cardiomyopathy Therapeutics Market: Qualitative Analysis
We perform Qualitative and Hypertrophic Cardiomyopathy Therapeutics Market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Hypertrophic Cardiomyopathy Treatment Market Landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Hypertrophic Cardiomyopathy Therapeutics Market Access and Reimbursement
The high cost of therapies for treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Hypertrophic Cardiomyopathy Therapeutics Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Hypertrophic Cardiomyopathy Therapeutics Market Report Scope
- The Hypertrophic Cardiomyopathy therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview of Hypertrophic Cardiomyopathy, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging Hypertrophic Cardiomyopathy therapies and the elaborate profiles of late-stage and prominent therapies will impact the current Hypertrophic Cardiomyopathy Treatment Market Landscape.
- A detailed review of the Hypertrophic Cardiomyopathy Therapeutics Market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Hypertrophic Cardiomyopathy Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Hypertrophic Cardiomyopathy market.
Hypertrophic Cardiomyopathy Therapeutics Market Report Insights
- Hypertrophic Cardiomyopathy Patient Population
- Hypertrophic Cardiomyopathy Therapeutic Approaches
- Hypertrophic Cardiomyopathy Pipeline Drugs Analysis
- Hypertrophic Cardiomyopathy Market Size
- Hypertrophic Cardiomyopathy Market Trends
- Existing and Future Hypertrophic Cardiomyopathy Market Opportunities
Hypertrophic Cardiomyopathy Treatment Market Report Key Strengths
- 11 years Hypertrophic Cardiomyopathy Market Forecast
- The 7MM Coverage
- Hypertrophic Cardiomyopathy Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Hypertrophic Cardiomyopathy Drugs Uptake
- Key Hypertrophic Cardiomyopathy Market Forecast Assumptions
Hypertrophic Cardiomyopathy Treatment Market Report Assessment
- Current Hypertrophic Cardiomyopathy Treatment Practices
- Hypertrophic Cardiomyopathy Unmet Needs
- Hypertrophic Cardiomyopathy Pipeline Drugs Analysis Profiles
- Hypertrophic Cardiomyopathy Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Hypertrophic Cardiomyopathy Market Insights
- What was the Hypertrophic Cardiomyopathy drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Hypertrophic Cardiomyopathy market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Hypertrophic Cardiomyopathy market size during the forecast period (2024–2034)?
- At what CAGR, the Hypertrophic Cardiomyopathy market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Hypertrophic Cardiomyopathy market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Hypertrophic Cardiomyopathy market growth till 2034 and what will be the resultant Hypertrophic Cardiomyopathy market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the Hypertrophic Cardiomyopathy market dynamics and subsequent analysis of the associated trends?
Hypertrophic Cardiomyopathy Epidemiology Insights
- What are the disease risk, burden, and unmet needs of Hypertrophic Cardiomyopathy?
- What is the historical Hypertrophic Cardiomyopathy patient population in the United States, EU4 (Germany, France, Italy, Spain) and the UK, and Japan?
- What would be the forecasted patient population of Hypertrophic Cardiomyopathy at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Hypertrophic Cardiomyopathy?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Hypertrophic Cardiomyopathy during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Treatment Scenario, Marketed Drugs, and Emerging Hypertrophic Cardiomyopathy Therapies
- What are the current options for the treatment of Hypertrophic Cardiomyopathy along with the approved therapy?
- What are the current treatment guidelines for the treatment of Hypertrophic Cardiomyopathy in the US, Europe, And Japan?
- What are the Hypertrophic Cardiomyopathy-marketed drugs and their Hypertrophic Cardiomyopathy MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many Hypertrophic Cardiomyopathy Companies are developing therapies for the treatment of Hypertrophic Cardiomyopathy?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Hypertrophic Cardiomyopathy?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to the Hypertrophic Cardiomyopathy therapies?
- What are the recent therapies, targets, Hypertrophic Cardiomyopathy Mechanisms of Action, and technologies developed to overcome the limitations of existing therapies?
- What are the Hypertrophic Cardiomyopathy Clinical Trials going on and their status?
- What are the key designations that have been granted for the emerging Hypertrophic Cardiomyopathy therapies?
- What are the 7MM historical and forecasted Hypertrophic Cardiomyopathy Drugs Market?
Reasons to Buy
- The Hypertrophic Cardiomyopathy therapeutics market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Hypertrophic Cardiomyopathy Drugs Market.
- Insights on patient burden/disease: Hypertrophic Cardiomyopathy Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Hypertrophic Cardiomyopathy drugs market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong Hypertrophic Cardiomyopathy Companies in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Hypertrophic Cardiomyopathy companies can strengthen their development and launch strategy.
Stay updated with us for Recent Articles