Idiopathic Pulmonary Fibrosis Market
- The total market size of Idiopathic Pulmonary Fibrosis in the 7MM was approximately USD 3,300 million in 2023 and is projected to increase during the forecast period (2024-2034).
- At the American Thoracic Society (ATS) 2024 conference, key presentations on idiopathic pulmonary fibrosis (IPF) included biomarker development, methods of differentiating fibrotic hypersensitivity pneumonitis from IPF, analyzing circulating proteomes associated with IPF as well as predicting disease type and severity, and the role of CPET in IPF prognosis
- Results presented on MN-001 (tipelukast) at the ATS 2024 demonstrated its significant potential in IPF in preclinical models due to its anti-inflammatory and anti-fibrotic properties. The study has been successfully completed, and ongoing analysis aims to thoroughly evaluate MN-001's effects on disease progression and patient well-being.
- The total market size of the Idiopathic Pulmonary Fibrosis treatment market is anticipated to experience growth during the forecast period (2024-2032) due to emerging treatments that includes Pamrevlumab, Tyvaso (treprostinil), PLN-74809, BI 1015550, VP01 (C21), BMS-986278, among others.
- The major players which are expected to launch their drug in the market during the forecast period of 2024-2034 include FibroGen, United Therapeutics, Pliant Therapeutics, Boehringer Ingelheim, Vicore Pharma, Bristol-Myers Squibb, to name a few.
DelveInsight’s report titled “Idiopathic Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of burns, historical and forecasted epidemiology, as well as the Idiopathic Pulmonary Fibrosis therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The report examines current treatment methodologies and algorithms for Idiopathic Pulmonary Fibrosis, assessing the overall market potential, identifying business prospects, and addressing pertinent unmet medical requirements.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and UK, Japan |
|
Idiopathic Pulmonary Fibrosis Market |
|
|
Idiopathic Pulmonary Fibrosis Market Size | |
|
Idiopathic Pulmonary Fibrosis Companies |
FibroGen, Hoffmann-La Roche Ltd, United Therapeutics, Boehringer Ingelheim, Pliant Therapeutics Inc., Galecto Biotech, Horizon Therapeutics, CSL Behring, Kadmon Corporation LLCs, MediciNova, PureTech, Bristol-Myers Squibb, Nitto Denko Corporation, Vicore Pharma AB, and others. |
|
Idiopathic Pulmonary Fibrosis Epidemiology Segmentation |
|
Idiopathic Pulmonary Fibrosis Treatment Market
Idiopathic Pulmonary Fibrosis Overview
The most prevalent type of pulmonary fibrosis is idiopathic pulmonary fibrosis that produces scarring (fibrosis). The term "idiopathic" refers to a condition that has no known cause. Scarring produces stiffness in the lungs, making breathing harder.
Idiopathic Pulmonary Fibrosis causes irreversible and progressive lung damage, which means it becomes worse over time. Certain drugs can help to slow it down in some circumstances. Lung transplantation is occasionally indicated for persons with idiopathic pulmonary fibrosis.
The signs and symptoms of idiopathic pulmonary fibrosis appear gradually and may not show up until the disease has caused significant lung damage. These are likely to deteriorate over time if they occur. Shortness of breath (dyspnea), dry coughing which eventually leads to chronic coughing in about 85% of the people with idiopathic pulmonary fibrosis are some of the most common signs and symptoms of idiopathic pulmonary fibrosis.
Idiopathic Pulmonary Fibrosis Diagnosis
There are several diagnostic tools available and the consensus guidelines have been well defined to identify idiopathic pulmonary fibrosis. Pulmonary function tests are performed to assess for restrictive lung disease which is characterized by decreased lung volumes (especially decreased forced vital capacity, total lung capacity, and functional residual capacity) and decreased diffusion capacity.
When idiopathic pulmonary fibrosis is suspected, laboratory tests to exclude autoimmune disease are also performed. Chest imaging is like x-rays is done but when they are not detailed enough to confirm idiopathic pulmonary fibrosis. High-resolution CT (HRCT) of the chest is performed. Patients may also be referred to a surgeon for a lung biopsy under general anesthesia in some instances.
Further details related to country-based variations are provided in the report...
Idiopathic Pulmonary Fibrosis Treatment
The therapeutic approach of idiopathic pulmonary fibrosis involves both nonpharmacological and pharmacological strategies. The goal of the treatment is to slow disease progression, reduce symptoms, prevent acute exacerbations, and prolong the survival. There are two antifibrotic agents ESBRIET (Roche) and OFEV (Boehringer Ingelheim) approved for use in idiopathic pulmonary fibrosis. Both drugs are known to slow the disease progression but do not significantly impact mortality.
There are several limitations with the current treatment regime, like adverse events related to gastrointestinal tract. Some of the most promising drugs which are currently under development includes potential drugs like Pamrevlumab (FibroGen), Tyvaso (Inhaled Treprostinil) (United Therapeutics), C21 (Vicore Pharma), and others covering the severity segments of Idiopathic Pulmonary Fibrosis are expected to drive the market during 2024-2034.
Idiopathic Pulmonary Fibrosis Epidemiology
As the market is derived using a patient-based model, the Idiopathic Pulmonary Fibrosis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis, gender-specific diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis, age-specific diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis, and severity-specific diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis in the 7MM covering the US, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
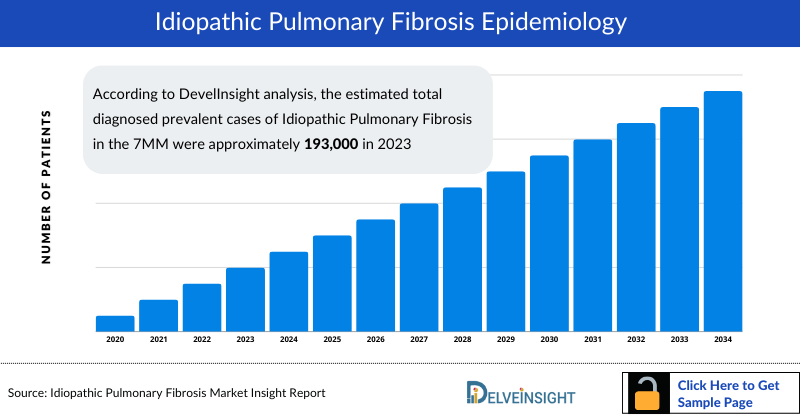
- According to DevelInsight analysis, the estimated total diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis in the 7MM were approximately 193,000 in 2023; this number is expected to increase in the future due to aging populations and improved diagnostic techniques.
- In 2023, among the 7MM, the US accounted for the highest diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis with approximately 95,000 cases. These cases are expected to increase during the forecast period (2024-2034).
- Among the EU4 and the UK, Germany had the highest diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis with nearly 21,000 cases, followed by the UK, and France with approximately 15,700, and 15,500 diagnosed prevalent cases respectively.
- In 2023, the prevalence of idiopathic pulmonary fibrosis in the 7MM was distributed as follows: mild (FVC >75%) with 55,926 cases, moderate (FVC 50%-75%) with 96,424 cases, and severe (FVC <50%) with 40,498 cases. This data underscores the significant number of patients in the moderate stage, indicating a critical need for effective management strategies.
- In the epidemiological model of Idiopathic Pulmonary Fibrosis, cases in the 7MM for 2023 are categorized into age groups with 6,573 cases in the 18-39 years group, 24,492 in the 40-59 years group, 113,411 in the 60-79 years group, and 48,368 in those over 80 years, indicating a significant increase in prevalence with age.
- In 2023, gender-specific diagnosed prevalent cases of Idiopathic Pulmonary Fibrosis in the 7MM were higher in males, with approximately 120,718 cases, compared to females, who had around 73,152 cases, reflecting a higher prevalence in the male population.
Idiopathic Pulmonary Fibrosis Recent Developments
- In October 2025, the FDA approved Boehringer Ingelheim’s JASCAYD® (nerandomilast), the first and only preferential PDE4B inhibitor, for oral treatment of idiopathic pulmonary fibrosis (IPF) in adults. Clinical trials showed JASCAYD slows lung function decline with a well-tolerated safety profile, offering a new antifibrotic and immunomodulatory therapy option.
- In February 2025, Elixirgen Therapeutics announced that the FDA granted Orphan Drug Designation to EXG-34217, a gene therapy for treating Telomere Biology Disorders (TBDs).
Idiopathic Pulmonary Fibrosis Drug Chapters
The drug chapter segment of the Idiopathic Pulmonary Fibrosis report encloses a detailed analysis of Idiopathic Pulmonary Fibrosis-marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the Idiopathic Pulmonary Fibrosis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Idiopathic Pulmonary Fibrosis Marketed Drugs
OFEV (Nintedanib): Boehringer Ingelheim Pharma GmbH
OFEV is a prescription drug for the treatment of IPF in adults. Nintedanib is the key ingredient and is a kinase inhibitor that inhibits multiple receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). Nintedanib is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). Nintedanib inhibits the following RTKs: platelet-derived growth factor receptor (PDGFR) a and ß, fibroblast growth factor receptor (FGFR) 1-3, vascular endothelial growth factor receptor (VEGFR) 1-3, and Fms-like tyrosine kinase-3 (FLT3).
The drug was approved by the FDA in 2014 whereas in the EU it was granted marketing authorization in 2015. In 2014, OFEV was granted Breakthrough Therapy designation during its review by the FDA.
Note: Further marketed drugs and their details will be provided in the report...
Idiopathic Pulmonary Fibrosis Emerging Drugs
BI 1015550: Boehringer Ingelheim
BI 1015550 is an investigational, oral phosphodiesterase 4B (PDE4B) inhibitor with combined antifibrotic and anti-inflammatory effects developed by Boehringer Ingelheim. BI 1015550 was studied as a monotherapy or in combination with background antifibrotic therapy to assess the effectiveness of slowing the rate of lung function decline in patients with Idiopathic Pulmonary Fibrosis.
The accelerated development of BI 1015550 is part of Boehringer Ingelheim’s next wave of potential innovative treatments for interstitial lung diseases aimed at preserving lung function and improving the lives of patients
In February 2022, Boehringer Ingelheim announced that the US FDA had granted Breakthrough Therapy Designation (BTD) to its novel investigational therapy, BI 1015550, for the treatment of idiopathic pulmonary fibrosis.
Note: Further emerging therapies and their detailed assessment will be provided in the final report...
Drug Class Insights
There's currently no cure for idiopathic pulmonary fibrosis. The main aim of treatment is to relieve the symptoms as much as possible and slow down its progression.
There are two medicines that can help slow down the progression of idiopathic pulmonary fibrosis in people: ESBRIET (Pirfenidone) and OFEV (Nintedanib). Both drugs have shown to slow the disease progression but not significantly impact mortality. For this reason, early initiation of therapy is recommended. Further studies also shown decreased exacerbations of idiopathic pulmonary fibrosis with these drugs. Serial monitoring of liver function tests is recommended while on either drug. The most common side effect reported with Nintedanib is diarrhea and with pirfenidone rash, photosensitivity, and gastrointestinal discomfort. Gastrointestinal side effects are the most common reason for discontinuing both drugs.
Before May 2022, ESBRIET and OFEV were the only approved rulers in market of idiopathic pulmonary fibrosis but recently Sandoz Pharmaceutical has launched the generic of pirfenidone in the US market. This entry of generic will put huge impact on the market of Hoffmann-La Roche’s ESBRIET and also will disturb the market of OFEV. For now, OFEV is one of the highest revenue generating product for Boehringer Ingelheim.
Idiopathic Pulmonary Fibrosis Market Outlook
There are two antifibrotic agents approved for use in idiopathic pulmonary fibrosis. These are Pirfenidone and Nintedanib (tyrosine kinase inhibitors). Both drugs are known to slow the disease progression but not significantly impact mortality. For this reason, early initiation of therapy is recommended. Further studies have also decreased exacerbations of idiopathic pulmonary fibrosis with these drugs. Serial monitoring of liver function tests is recommended while on either drug. The most common side effect reported with Nintedanib is diarrhea and pirfenidone rash, photosensitivity, and gastrointestinal discomfort. Gastrointestinal side effects are the most common reason for discontinuing both drugs. According to the current survey reports, most European physicians are either unaware of these antifibrotic drugs or believe in the wait and watch strategies during the progression of idiopathic pulmonary fibrosis. It was found that only 71% of mild idiopathic pulmonary fibrosis diagnosed patients, 41% of moderate idiopathic pulmonary fibrosis diagnosed patients, and around 60% of severe idiopathic pulmonary fibrosis diagnosed patients receive treatment in European counties.
Until 2014, in the US, the standard practice for treating idiopathic pulmonary fibrosis focused primarily on immunosuppressant therapy using a combination of prednisone, azathioprine, and N-acetylcysteine.
Nintedanib or pirfenidone are good choices as the primary treatment for idiopathic pulmonary fibrosis at present. But, neither treatment has been shown to completely cure the disease and thus there is room for improved treatments that address the cause of the condition. Also, these drugs may incur high out-of-pocket costs without changing the overall progression of the disease and the high mortality within 3–5 years after diagnosis.
In the emerging scenario, several companies are creating medications to serve better treatment therapies for idiopathic pulmonary fibrosis and are expected to enter the market including Pamrevlumab (FibroGen), Tyvaso (treprostinil) (United Therapeutics), PLN-74809 (Pliant Therapeutics, Inc.), BI 1015550 (Boehringer Ingelheim), VP01 (C21) (Vicore Pharma AB), BMS-986278 (Bristol-Myers Squibb), among others. They aim to investigate their products for the treatment of Idiopathic Pulmonary Fibrosis.
According to DelveInsight, the overall dynamics of the Idiopathic Pulmonary Fibrosis market are anticipated to change in the coming years owing to the expected launch of emerging therapies.
- The total market size of Idiopathic Pulmonary Fibrosis in the 7MM was approximately USD 3,300 million in 2023 and is projected to increase during the forecast period (2024-2034).
- DelveInsight’s analysts estimate that the market is expected to show positive growth, mainly attributed to the increase in population and also, and the launch of upcoming therapies during the forecast period (2024-2034).
- The Idiopathic Pulmonary Fibrosis market size in the EU4 and the UK was nearly USD 702 million and accounted for nearly 21% of the total 7MM market size in 2023.
- Among the EU4 and the UK, Germany holds the highest market size of around USD 183 million followed by the UK, and France with approximately USD 141 million, and USD 140 million respectively. These numbers are expected to change during the forecast period.
- The Idiopathic Pulmonary Fibrosis market size in Japan accounted for nearly 5% of the total 7MM market size in 2023, these numbers are expected to rise by 2034.
Continued in report...
Idiopathic Pulmonary Fibrosis Drugs Uptake
This section focuses on the rate of uptake of the potential drugs expected to get launched in the market during the study period 2020-2034. For example, for Pamrevlumab, we expect the drug uptake to be medium with a peak share of 7%, years to peak is expected to be 7 years from the year of launch.
Further detailed analysis of emerging therapies drug uptake in the report...
Idiopathic Pulmonary Fibrosis Pipeline Development Activities
The report provides insights into different Idiopathic Pulmonary Fibrosis clinical trails within in Phase III. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing and patent details for Idiopathic Pulmonary Fibrosis emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Idiopathic Pulmonary Fibrosis evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others. DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Medical University of South Carolina, Children’s Hospital of Richmond, University of Edinburgh, Imperial College London, Hospital San Carlo, Universität Bonn, and Nagoya City University Graduate School of Medical Sciences, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Idiopathic Pulmonary Fibrosis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
According to our primary research analysis, despite considerable progress in Idiopathic Pulmonary Fibrosis treatment one of the primary challenges associated with Idiopathic Pulmonary Fibrosis, according to physicians, is the progressive decline in lung function. Idiopathic Pulmonary Fibrosis is a complex and heterogeneous disease, with significant variability in disease severity and progression among patients. Physicians emphasize the need for personalized treatment approaches tailored to individual patient characteristics, including genotype, phenotype, and disease stage.
According to a KOL in the US, even though numerous innovative treatments are currently undergoing preclinical and clinical testing, some limiting factors such as mutation class, genetic profile, drug interactions, adverse effects, and cost remain the major areas of concern. Along with this, safe and efficient disease-modifying agents are still required in the treatment paradigm which serves as the cure for the disease.
As per another KOL, in the past, Idiopathic Pulmonary Fibrosis was a digestive and lung condition that mostly affected young children. However, in recent years, the condition has been seen to be causing multi-system complications in adults. The disease is now affecting more adults as compared to children due to a decline in its mortality rate.
In another KOL in Japan, there are high numbers of Idiopathic Pulmonary Fibrosis patients with F508del mutation in the American or European population but CFTR mutations in the Japanese population are somewhat different where F508 variants are observed infrequently.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in Idiopathic Pulmonary Fibrosis trials, one of the most important primary outcome measures is complete eschar removal.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The reimbursement challenges related to medical care and treatment for individuals with Idiopathic Pulmonary Fibrosis can be significant as it often requires specialized medical attention, covering the costs of diagnosis, treatment, and ongoing care. Health insurance plans may not fully cover limited coverage of some medical treatments, and therapies specific to cervical dystonia. This can result in high out-of-pocket expenses for families seeking the best care for their loved ones. Moreover, it requires specialized care from healthcare providers with expertise. Finding and accessing such specialists may be challenging, and the associated costs may not always be fully reimbursed by insurance.
Further details will be provided in the report...
Scope of the Idiopathic Pulmonary Fibrosis Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Idiopathic Pulmonary Fibrosis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current treatment landscape.
- A detailed review of the Idiopathic Pulmonary Fibrosis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Idiopathic Pulmonary Fibrosis market.
Idiopathic Pulmonary Fibrosis Report Insights
- Idiopathic Pulmonary Fibrosis Patient Population
- Idiopathic Pulmonary Fibrosis Therapeutic Approaches
- Idiopathic Pulmonary Fibrosis Pipeline Analysis
- Idiopathic Pulmonary Fibrosis Market Size and Trends
- Existing and Future Market Opportunity
Idiopathic Pulmonary Fibrosis Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Idiopathic Pulmonary Fibrosis Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Idiopathic Pulmonary Fibrosis Drugs Uptake
- Key Idiopathic Pulmonary Fibrosis Market Forecast Assumptions
- Idiopathic Pulmonary Fibrosis Report Assessment
Current Idiopathic Pulmonary Fibrosis Treatment Practices
- Idiopathic Pulmonary Fibrosis Unmet Needs
- Idiopathic Pulmonary Fibrosis Pipeline Product Profiles
- Idiopathic Pulmonary Fibrosis Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
- Idiopathic Pulmonary Fibrosis Market Drivers
- Idiopathic Pulmonary Fibrosis Market Barriers
Key Questions Answered In The Idiopathic Pulmonary Fibrosis Market Report
Idiopathic Pulmonary Fibrosis Market Insights
- What was the Idiopathic Pulmonary Fibrosis total market size, market size by therapies, market share (%) distribution in 2019 and how it would all look like in 2032? What are the contributing factors for this growth?
- How are different therapies going to affect the treatment paradigm of Idiopathic Pulmonary Fibrosis?
- What kind of uptake Pamrevlumab, Tyvaso (treprostinil), PLN-74809, BI 1015550, VP01 (C21), BMS-986278 will witness in the coming 10 years?
- Which drug is going to be the largest contributor by 2032?
- What are the pricing variations among different geographies for approved and other therapies?
- How would the market drivers, barriers and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Idiopathic Pulmonary Fibrosis Epidemiology Insights
- What is the disease risk, burden, and unmet needs of Idiopathic Pulmonary Fibrosis?
- How will the prevalent population of the indication change during the forecast period?
- What is the historical and forecasted Idiopathic Pulmonary Fibrosis patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- What is the prevalence rate of Idiopathic Pulmonary Fibrosis and what are the evidence from literature or primary market research related to this?
- What factors are affecting the increase in prevalent cases of Idiopathic Pulmonary Fibrosis?
Current Idiopathic Pulmonary Fibrosis Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Idiopathic Pulmonary Fibrosis? What are the current guidelines for treating Idiopathic Pulmonary Fibrosis in the US and Europe?
- How many companies are developing therapies for the treatment of Idiopathic Pulmonary Fibrosis?
- How many emerging therapies are in the mid-stage and late stage of development for treating Idiopathic Pulmonary Fibrosis?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of approved therapies?
- What is the 7MM historical and forecasted market of Idiopathic Pulmonary Fibrosis?
Reasons to Buy Idiopathic Pulmonary Fibrosis Market Forecast Report:
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Idiopathic Pulmonary Fibrosis therapeutics market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies for stuttering, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
For More In-depth Information @ Latest DelveInsight Blog