Lennox Gastaut Syndrome Market
- In 2023, DelveInsight estimated approximately 81 thousand diagnosed prevalent cases of Lennox Gastaut Syndrome (Lennox Gastaut Syndrome) across the 7MM. The United States accounted for 42.6% of these cases, with the EU4 and the UK comprising around 53.7%, and Japan contributing about 3.8%.
- The Lennox Gastaut Syndrome market is set for steady growth, with a strong compound annual growth rate (CAGR) projected from 2024 to 2034. This expansion across the 7MM will be fueled by the introduction of innovative therapies, including EPX-100, Carisbamate, among others. Additionally, greater awareness of Lennox Gastaut Syndrome, is leading to earlier diagnosis and better management, driving demand for effective therapies, further increasing the market for Lennox Gastaut Syndrome.
- According to DelveInsight’s analysis, over the forecast period from 2024 to 2034, the Lennox Gastaut Syndrome market is projected to grow at a CAGR of 4.5%.
- Several new therapies targeting seizure control in Lennox Gastaut Syndrome, such as EPIDIOLEX (cannabidiol) and FINTEPLA (fenfluramine) have gained regulatory approvals, offering more treatment options. These therapies address unmet needs in patients who are resistant to traditional antiepileptic drugs which will further drive the market size.
- Lennox Gastaut Syndrome treatment faces challenges like poor seizure control, with many patients showing drug resistance. Existing therapies often fail to manage all seizure types, and side effects like cognitive decline and sedation further limit effectiveness. There is also a lack of long-term safety data, making the need for more targeted and tolerable treatments essential.
DelveInsight’s “Lennox Gastaut Syndrome Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Lennox Gastaut Syndrome, historical and forecasted epidemiology, as well as the Lennox Gastaut Syndrome therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Lennox Gastaut Syndrome market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Lennox Gastaut Syndrome market size from 2020 to 2034. The report also covers Lennox Gastaut Syndrome treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Lennox Gastaut Syndrome Market |
|
|
Lennox Gastaut Syndromes Market Size | |
|
Lennox Gastaut Syndrome Companies |
GlaxoSmithKline, Meda Pharmaceuticals, Roche, Lundbeck, Greenwich Biosciences, Janssen Pharmaceuticals, Eisai, Zogenix, Takeda, Ovid Therapeutics, and others. |
|
Lennox Gastaut Syndrome Epidemiology Segmentation |
|
Lennox Gastaut Syndrome Treatment Market
Lennox Gastaut Syndrome overview
Lennox Gastaut Syndrome is a severe pediatric epilepsy syndrome characterized by the presence of multiple pharmaco-resistant seizure types, including tonic, atypical absences, and tonic or atonic drop attacks, and the presence of electroencephalographic abnormalities, such as slow-spike waves and paroxysmal fast rhythms. Intellectual disability, behavioral and psychiatric disorders are common comorbidities; and these disturbances have multi-factorial pathogenesis.
In addition to the classic seizures, patients with Lennox Gastaut Syndrome can experience many other seizures, such as myoclonic, focal, and non-convulsive status epilepticus, especially in later stages of the disease. Although intellectual disability (ID) is seen in most patients, nevertheless it can also be absent; therefore, ID is not to be considered as a diagnostic criterion
Lennox Gastaut Syndrome diagnosis
Diagnosis of Lennox Gastaut Syndrome is usually made based upon thorough clinical evaluation, detailed patient history, and a complete physical and neurological evaluation including advanced imaging techniques, such as electroencephalography (EEG) and magnetic resonance imaging (MRI).
Lennox Gastaut Syndrome can be differentiated from other epilepsy syndromes based on history and EEG characteristics, but achieving an accurate diagnosis can be challenging. Not all patients with Lennox Gastaut Syndrome display the characteristic triad of features, particularly at the onset. Despite clear parameters and distinguishing clinical features, significant overlap exists between Lennox Gastaut Syndrome and other early-onset epileptic encephalopathies. Drop attacks, which are seen frequently in Lennox Gastaut Syndrome patients, occur in many other syndromes. Epilepsies that share this or other characteristics of Lennox Gastaut Syndrome include focal epilepsies with secondary bilateral synchrony, myoclonic-astatic epilepsy (Doose syndrome), Dravet syndrome, West syndrome, and atypical benign partial epilepsy of childhood.
Further details related to country-based variations are provided in the report...
Lennox Gastaut Syndrome treatment
Current treatment for Lennox-Gastaut Syndrome (Lennox Gastaut Syndrome) focuses on managing seizures, as there is no cure for the condition. Standard therapies include a combination of anti-epileptic drugs (AEDs) like valproate, lamotrigine, clobazam, and topiramate, which are used to reduce seizure frequency. Additionally, newer therapies like EPIDIOLEX (cannabidiol) and FINTEPLA (fenfluramine) have shown efficacy in treating seizures in Lennox Gastaut Syndrome patients. Vagus nerve stimulation (VNS), ketogenic diets, and surgical interventions are also considered when pharmacological treatments are insufficient. However, despite these options, most patients experience drug resistance, highlighting the need for more effective, targeted treatments.
Lennox Gastaut Syndrome Epidemiology
As the market is derived using a patient-based model, the Lennox Gastaut Syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total prevalent cases of Lennox Gastaut Syndrome, diagnosed prevalent cases of Lennox Gastaut Syndrome, gender-specific diagnosed prevalent cases of Lennox Gastaut Syndrome, seizure -specific diagnosed prevalent cases of Lennox Gastaut Syndrome in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
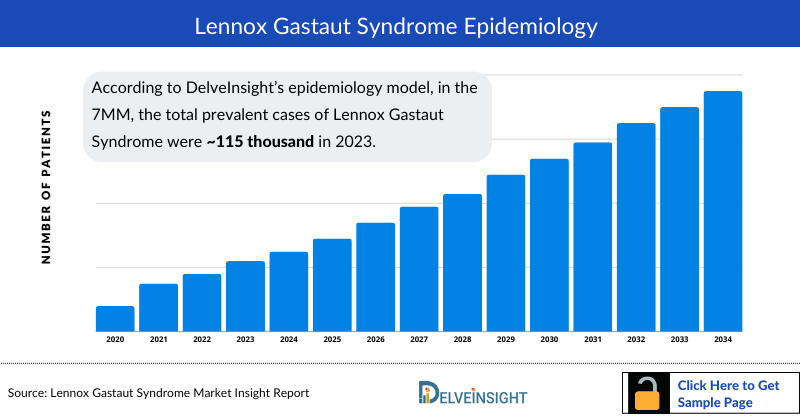
- According to DelveInsight’s epidemiology model, in the 7MM, the total prevalent cases of Lennox Gastaut Syndrome were approximately 115 thousand in 2023. This number is anticipated to rise during the forecast period (2024-2034), driven by increased awareness and improved diagnostic techniques.
- In 2023, the total number of diagnosed prevalent cases of Lennox Gastaut Syndrome across the 7MM was 81 thousand. This figure is anticipated to rise further over the forecast period.
- In 2023, the US accounted for the highest number of diagnosed prevalent cases of Lennox Gastaut Syndrome, with approximately 34 thousand cases which is further expected to increase during the forecasted period.
- In 2023, within the US, the diagnosed prevalent cases of Lennox Gastaut Syndrome were divided based on gender with males contributing approximately 25 thousand cases, and females accounting for around 9 thousand cases.
- In 2023, among the EU4 and the UK, Germany had the highest number of diagnosed prevalent cases of Lennox Gastaut Syndrome, with approximately 11 thousand cases. France followed with nearly 9.6 thousand cases, and Italy with around 8.8 thousand cases.
- In 2023, Japan accounted for the lowest number of diagnosed prevalent cases of Lennox Gastaut Syndrome, with approximately 3 thousand cases.
Lennox Gastaut Syndrome Drug Chapters
Lennox Gastaut Syndrome Marketed Drug
FINTEPLA (fenfluramine): UCB
FINTEPLA (fenfluramine) oral solution is a prescription medication approved by the FDA, EU Commission, and PMDA for the treatment of seizures associated with Lennox Gastaut Syndrome as an add-on therapy to other anti-epileptic medicines for patients 2 years of age and older.
Fenfluramine is a serotonin releasing agent, and thereby stimulates multiple 5-HT receptor sub-types through the release of serotonin. Fenfluramine may reduce seizures by acting as an agonist at specific serotonin receptors in the brain, including the 5-HT1D, 5-HT2A, and 5-HT2C receptors, and also by acting on the sigma-1 receptor.
EPIDIOLEX (cannabidiol): Jazz Pharmaceuticals
It is the first plant-derived cannabinoid prescription medicine approved in the US EPIDIOLEX works by targeting seizure activity, although its precise mechanism in Lennox Gastaut Syndrome is not fully understood.
The drug has shown significant efficacy in reducing the frequency of seizures in clinical trials and is generally well-tolerated, with side effects such as drowsiness, diarrhea, and decreased appetite. It is a valuable treatment option for Lennox Gastaut Syndrome patients who often experience drug-resistant seizures, offering an additional option when conventional therapies are ineffective.
Lennox Gastaut Syndrome Emerging Drugs
EPX-100: Epygenix
The lead drug candidate is currently advancing through a Phase II clinical trial for Lennox Gastaut Syndrome. Its safety profile has been thoroughly established through comprehensive preclinical IND-enabling toxicology, chronic toxicology, and pharmacology studies. Previously approved by the FDA as a first-generation antihistamine, the candidate has demonstrated an excellent safety profile. EPX-100, acts as a potent serotonergic agonist, targeting 5HT2B, 5HT2C, and 5HT2A receptors, positioning it as a strong contender for seizure disorder therapies.
Carisbamate: SK Life Science
Carisbamate was discovered by SK Biopharmaceuticals and is being developed by SK life science for the potential treatment of Lennox Gastaut Syndrome. While the precise mechanism by which carisbamate exerts its therapeutic effect is unknown, it is believed to reduce repetitive neuronal firing by inhibiting voltage-gated sodium currents. Carisbamate has received orphan drug designation for the potential treatment of Lennox Gastaut Syndrome from the US Food and Drug Administration (FDA).
Note: Further emerging therapies and their detailed assessment will be provided in the final report...
Drug Class Insights
For Lennox Gastaut Syndrome, the primary drug class is Antiepileptic Drugs (AEDs), such as valproate, lamotrigine, and topiramate, which work to stabilize neuronal activity and reduce seizure frequency. Cannabinoids, notably EPIDIOLEX (cannabidiol), offer another approach by modulating the endocannabinoid system to address severe seizure forms. Additionally, while not a drug, the ketogenic diet is an important treatment modality that, when used alongside pharmacotherapy, can help control seizures. These treatments each target different mechanisms to manage the complex seizure patterns characteristic of Lennox Gastaut Syndrome.
Continued in report…
Lennox Gastaut Syndrome Market Outlook
The market for Lennox Gastaut Syndrome is evolving with a focus on addressing unmet needs in treatment efficacy and safety. The current market is dominated by established antiepileptic drugs (AEDs) like valproate, lamotrigine, and topiramate, which are foundational but often inadequate for refractory cases. The introduction of cannabinoids, notably EPIDIOLEX (cannabidiol), has added a significant option, targeting severe and drug-resistant seizures with a different mechanism of action.
The pipeline is promising, with several new drug candidates and treatment modalities in development. The market is also seeing increased interest due to the high unmet need for more effective therapies, given the limited options for patients with refractory Lennox Gastaut Syndrome.
Regulatory designations like Orphan Drug and Rare Pediatric Disease status are enhancing the market potential for new therapies, encouraging investment and innovation. Overall, the Lennox Gastaut Syndrome market is characterized by a high demand for novel treatments that offer better seizure control and improved quality of life for patients.
Continued in report...
Lennox Gastaut Syndrome Drugs Uptake
This section focuses on the uptake rate of potential Lennox Gastaut Syndrome drugs expected to be launched in the market during 2020–2034. For example EPX-100 is expected to enter the US market in XXX and is projected to have a XXX uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Lennox Gastaut Syndrome Pipeline Development Activities
The report provides insights into different Lennox Gastaut Syndrome clinical trials within Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Lennox Gastaut Syndrome.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Lennox Gastaut Syndrome evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Massachusetts General Hospital, Vanderbilt University Medical Center, Louisiana State University, LOEWE Center for Personalized and Translational Epilepsy Research (CePTER), Paediatric Neurosciences Research Group, Royal Hospital for Children, School of Health and Wellbeing, University of Glasgow, Glasgow, UK, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa, Genova, Italy, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Lennox Gastaut Syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
Lennox-Gastaut usually has 3 or more of the major and 3 or more of the minor. So with that simple thing that we actually check by medical students reviewing records, it’s very reliable. You don’t have to be an epileptologist to say this is possibly Lennox-Gastaut.
The only three essential factors of Lennox-Gastaut are having an etiology for epilepsy, any of them; refractoriness early on, not forever but early on; and a childhood onset, of course. If you have those 3, you don’t have to have any gene that makes you have Lennox-Gastaut. There may be some genes that may make you more prone to development, but that’s what you need.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The EPIDIOLEX Copay Savings Program offers the first 30 days of EPIDIOLEX for as low as USD 0 and refills for as low as USD 25 per month. The program is only for commercially insured Epidiolex patients with a legal residence in the United States. Qualifying patients may receive up to USD 3,000 annually to help meet co-pay costs. Co-pay savings are applied directly to the pharmacy.
In the US, around 97% of all US insured patients have Epidiolex coverage for Lennox Gastaut Syndrome and Dravet Syndrome. Currently, EPIDIOLEX is a preferred brand with the lowest brand co-pay, and company re-alignment of PBM clients creates commercial opportunities. GW pharmaceuticals continued partnerships with payers to expand access and enter the long-term care segment.
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further details will be provided in the report...
Scope of the Lennox Gastaut Syndrome Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Lennox Gastaut Syndrome explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Lennox Gastaut Syndrome market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Lennox Gastaut Syndrome market.
Lennox Gastaut Syndrome report insights
- Lennox Gastaut Syndrome Patient Population
- Lennox Gastaut Syndrome Therapeutic Approaches
- Lennox Gastaut Syndrome Pipeline Analysis
- Lennox Gastaut Syndrome Market Size and Trends
- Existing and Future Market Opportunity
Lennox Gastaut Syndrome report key strengths
- 11 years Forecast
- The 7MM Coverage
- Lennox Gastaut Syndrome Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Lennox Gastaut Syndrome Drugs Uptake
- Key Lennox Gastaut Syndrome Market Forecast Assumptions
Lennox Gastaut Syndrome report assessment
- Current Lennox Gastaut Syndrome Treatment Practices
- Lennox Gastaut Syndrome Unmet Needs
- Lennox Gastaut Syndrome Pipeline Product Profiles
- Lennox Gastaut Syndrome Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
- Lennox Gastaut Syndrome Market Drivers
- Lennox Gastaut Syndrome Market Barriers
Key Questions Answered In The Lennox Gastaut Syndrome Market Report
Lennox Gastaut Syndrome Market Insights
- What was the total market size of Lennox Gastaut Syndrome, the market size of Lennox Gastaut Syndrome by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will Carisbamate affect the treatment paradigm of Lennox Gastaut Syndrome?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Lennox Gastaut Syndrome Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Lennox Gastaut Syndrome? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Lennox Gastaut Syndrome?
- What is the historical and forecasted Lennox Gastaut Syndrome patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Lennox Gastaut Syndrome population during the forecast period (2024–2034)?
- What factors are contributing to the growth of Lennox Gastaut Syndrome cases?
Current Lennox Gastaut Syndrome Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Lennox Gastaut Syndrome? What are the current clinical and treatment guidelines for treating Lennox Gastaut Syndrome?
- How many companies are developing therapies for the treatment of Lennox Gastaut Syndrome?
- How many emerging therapies are in the mid-stage and late stage of development for treating Lennox Gastaut Syndrome?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of Lennox Gastaut Syndrome?
Reasons to Buy Lennox Gastaut Syndrome Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Lennox Gastaut Syndrome market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Lennox Gastaut Syndrome, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.