migraine pipeline insight
DelveInsight’s, “Migraine - Pipeline Insight, 2026,” report provides comprehensive insights about 30+ companies and 30+ pipeline drugs in Migraine pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Geography Covered
- Global coverage
Migraine Understanding
Migraine: Overview
Migraine is a neurological pain condition that affects millions of people. It is the most common neurological disease that has significant effects on the brain and consequently on behaviors associated with repeated migraine attacks. The stress of migraine is associated with premigraine, intermigraine, and postmigraine processes. Understanding of migraine includes how normally non-painful stimuli such as light may exacerbate the pain of migraine and how the migraine headache may produce changes in skin sensitivity (allodynia) that can involve the whole body. Alterations in the brain's function and structure, as revealed by advanced imaging techniques, have provided new insights into the mechanisms of the disease. The reach of migraine headaches spans the globe.1 Migraine is sometimes confused with other types of headache, such as tension headache. Those with migraines may not receive the correct diagnosis, adequate treatment, or proper support from family, friends, or coworkers. Migraine treatment usually consists of acute or abortive medications, whereas preventive medications are used by a minority of individuals with migraine. Migraines are a leading cause of disability and suffering worldwide. There are several risk factors associated with migraine occurrence. Nonmodifiable factors include genetics, gender, and age. The probability of migraines is 40% in a person with 1 parent with migraines and 75% if both parents experience migraines. Adult women are 3 times more likely than men to have migraines. However, in preadolescents, migraines are more common in boys. Migraines usually have an onset in late childhood/early adolescence, and the prevalence peaks in individuals in their 50s, with notable decreases as people enter their 60s and 70s and rare occurrences in people 80 years and older. Migraine triggers are patient specific. Examples include food additives, caffeine, artificial sweeteners, and delayed or missed meals. To determine the probability of an item being a trigger, patients should avoid the item for at least 4 weeks and then slowly reintroduce it, keeping in mind that migraines may start 24 to 48 hours before headache onset.
“Migraine- Pipeline Insight, 2026" report by DelveInsight outlays comprehensive insights of present scenario and growth prospects across the indication. A detailed picture of the Migraine pipeline landscape is provided which includes the disease overview and Migraine treatment guidelines. The assessment part of the report embraces, in depth Migraine commercial assessment and clinical assessment of the pipeline products under development. In the report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Migraine collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
Report Highlights
The companies and academics are working to assess challenges and seek opportunities that could influence Migraine R&D. The therapies under development are focused on novel approaches to treat/improve Migraine.
Migraine Emerging Drugs Chapters
This segment of the Migraine report encloses its detailed analysis of various drugs in different stages of clinical development, including phase II, I, preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
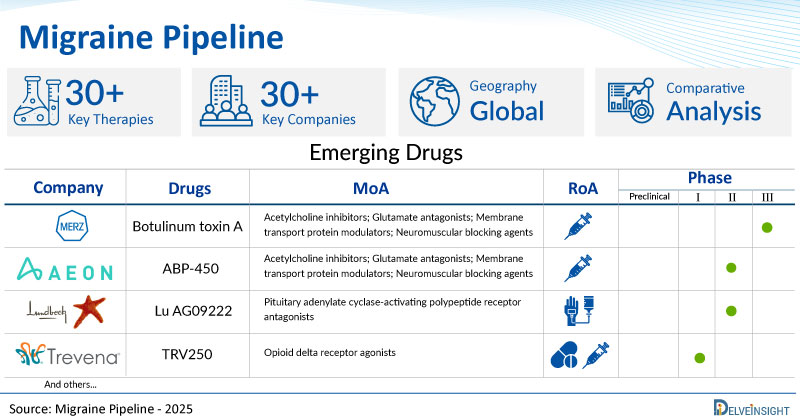
Migraine Emerging Drugs
AXS-07: Axsome Therapeutics
AXS-07 is a novel, oral, rapidly absorbed, multi-mechanistic, investigational medicine. AXS-07 consists of MoSEIC (Molecular Solubility Enhanced Inclusion Complex) meloxicam and rizatriptan. AXS-07 is thought to act by inhibiting Calcitonin gene-related peptide (CGRP) release, reversing CGRP-mediated vasodilation, and inhibiting neuroinflammation, pain signal transmission, and central sensitization. Meloxicam is a new molecular entity for migraine enabled by MoSEIC™ technology, which results in rapid absorption of meloxicam while maintaining a long plasma half-life. AXS-07 is currently being developed for the acute treatment of migraine. The product is in the NDA submitted phase of development.
STS-101: Satsuma Pharmaceuticals
STS101 combines the Satsuma powder technology with an easy-to-use nasal delivery device to create a reliable and convenient DHE product potentially able to provide the unique clinical advantages of DHE while overcoming the shortcomings of existing DHE products. TS101 has a number of key advantages that we believe may provide significant benefits over other acute treatments for migraine and result in robust and consistent clinical performance. These advantages arise from our proprietary dry-powder formulation, which incorporates a mucoadhesive drug carrier and engineered drug particle technologies, and our proprietary nasal delivery device. STS101 is an investigational product that is currently being evaluated in Phase 3 clinical trials for the acute treatment of migraine and is not approved by the U.S. Food and Drug Administration.
Zavegepant: Biohaven Pharmaceuticals
Zavegepant (BHV-3500) is a third generation, high affinity, selective and structurally unique, small molecule CGRP receptor antagonist. The chemical properties of zavegepant make the product candidate potentially suitable for multiple routes of delivery, including nasal, subcutaneous, inhalation or oral administration.It is currently in Phase III stage of development for Migraine and is being developed by Biohaven Pharmaceuticals.
TNX1900: Tonix Pharmaceuticals
TNX-1900(Oxytocin), Tonix’s proprietary potentiated intranasal oxytocin is in the pre-Investigational New Drug (IND) stage and is currently being studied as a candidate for prophylaxis of chronic migraine. Oxytocin is a naturally occurring human hormone that acts as a neurotransmitter in the brain. In clinical and preliminary research, it has been observed that increased oxytocin levels can relieve headaches. When oxytocin is delivered via the nasal route, it results in enhanced binding to receptors on neurons in the trigeminal system, inhibiting transmission of pain signals. Intranasal oxytocin has been well tolerated in several clinical trials in adults and children and has been shown to block calcitonin gene-related peptide (CGRP) release in animals, a pathway known to be critical to the pathogenesis of migraine attacks. TNX-1900 is believed to interrupt pain signals at the trigeminal ganglia by suppressing electrical impulses, a potentially different activity than drugs that just block CGRP. TNX-1900 is an investigational new drug and has not been approved for any indication.
Further product details are provided in the report……..
Migraine: Therapeutic Assessment
This segment of the report provides insights about the different Migraine drugs segregated based on following parameters that define the scope of the report, such as:
Major Players in Migraine
There are approx. 30+ key companies which are developing the therapies for Migraine. The companies which have their Migraine drug candidates in the most advanced stage, i.e. phase III include, Novartis.
Discover key insights into the Migraine market landscape! Explore emerging therapies, market trends, and future forecasts today.
Phases
DelveInsight’s report covers around 30+ products under different phases of clinical development like
- Late stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Route of Administration
Migraine pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
- Intra-articular
- Intraocular
- Intrathecal
- Intravenous
- Ophthalmic
- Oral
- Parenteral
- Subcutaneous
- Topical
- Transdermal
Molecule Type
Products have been categorized under various Molecule types such as
- Oligonucleotide
- Peptide
- Small molecule
Product Type
Drugs have been categorized under various product types like Mono, Combination and Mono/Combination.
Migraine: Pipeline Development Activities
The report provides insights into different therapeutic candidates in phase III, II, I, preclinical and discovery stage. It also analyses Migraine therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Migraine drugs.
Get a detailed overview of How the market will evolve in the upcoming years: Migraine Market
Migraine Report Insights
- Migraine Pipeline Analysis
- Therapeutic Assessment
- Unmet Needs
- Impact of Drugs
Migraine Report Assessment
- Pipeline Product Profiles
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Questions
Current Scenario and Emerging Therapies:
- How many companies are developing Migraine drugs?
- How many Migraine drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for Migraine?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Migraine therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Migraine and their status?
- What are the key designations that have been granted to the emerging drugs?