Molluscum Contagiosum Market
- With the Molluscum Contagiosum Prevalence increasing globally, there's a corresponding rise in demand for effective treatments. This surge in demand is influenced by factors such as population growth, increased awareness, and changing lifestyles, contributing to market growth opportunities.
- The Molluscum Contagiosum Treatment Market is promising, driven by factors such as increasing Molluscum Contagiosum Prevalence, rising awareness, expanding treatment options, and a competitive landscape. However, challenges such as regulatory hurdles and the need for continued innovation remain key considerations for stakeholders in this market.
- Current Molluscum Contagiosum Treatment include physical removal methods like cryotherapy and curettage, as well as topical medications such as imiquimod or cantharidin solution, aimed at lesion clearance and symptom alleviation.
- The US FDA-approved Molluscum Contagiosum Therapies encompass YCANTH (VP-102/cantharidin), a topical solution indicated for the Molluscum Contagiosum Treatment in patients 2 years of age and older, with common local skin reactions at the application site. On the other hand, ZELSUVMI (berdazimer) is a 10.3% gel approved for the treatment of molluscum contagiosum, offering a potential treatment option with a mechanism of action that involves targeted delivery through a single-use applicator for topical dosing.
- Unmet needs in Molluscum Contagiosum Treatment stem from the lack of consensus on clinical practice guidelines, necessitating more standardized approaches and novel therapeutic options to improve efficacy and patient outcomes. Addressing these gaps requires research into innovative treatments and the development of evidence-based guidelines to optimize management strategies.
- Molluscum Contagiosum is a self-limiting condition that often resolves on its own within 6-12 months, without the need for treatment. This natural resolution of the infection can reduce the perceived urgency for seeking medical intervention, potentially slowing market growth.
- However, the Molluscum Contagiosum Pipeline is limited, but Veloce Biopharma's VBP-245 holds promise in the treatment regime for molluscum contagiosum. VBP-245 is a topical gel, it is a resistance-free, broad-spectrum antimicrobial agent that eradicates micro-organisms, including bacteria, viruses, yeasts, molds, fungi, and protozoa.
Request for Unlocking the Sample Page of the "Molluscum Contagiosum Treatment Market"
DelveInsight’s “Molluscum Contagiosum Treatment Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of molluscum contagiosum, historical and forecasted epidemiology, as well as the molluscum contagiosum market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Molluscum Contagiosum Treatment Market report provides current treatment practices, emerging drugs, Molluscum Contagiosum Drugs Market Share of individual therapies, and current and forecasted 7MM molluscum contagiosum market size from 2020 to 2034. The report also covers Molluscum Contagiosum Treatment Market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024 to 2034 |
|
Geographies Covered |
|
|
Molluscum Contagiosum Drugs Market |
|
|
Molluscum Contagiosum Market Size | |
|
Molluscum Contagiosum Companies |
|
Molluscum Contagiosum Treatment Market
The molluscum contagiosum, also called water warts, is a common superficial skin infection caused by the poxvirus. It manifests as small, raised, round bumps or lesions on the skin, typically with a central indentation or core. These lesions are usually painless but may become itchy, inflamed, or irritated, especially if scratched or rubbed. The infection is caused by the molluscum contagiosum virus, which belongs to the poxvirus family.
It spreads through direct skin-to-skin contact with an infected person or by touching contaminated objects such as towels, clothing, or toys. The primary symptom is the appearance of small, flesh-colored, or pearly bumps on the skin, usually measuring 2 to 5 millimeters in diameter. These bumps may occur individually or in clusters and can develop anywhere on the body, but they are most commonly found in areas that are warm and moist, such as the armpits, groin, abdomen, and buttocks. Molluscum contagiosum is highly contagious. It can spread through close personal contact, sexual contact, sharing contaminated objects, or through contact with contaminated surfaces in public places such as swimming pools or gymnasiums.
Furthermore, individuals with weakened immune systems, such as those with HIV/AIDS or undergoing chemotherapy, are at higher risk of developing severe or widespread molluscum contagiosum infections. Children, particularly those in daycare settings or with frequent skin-to-skin contact, are also more susceptible.
Molluscum contagiosum Diagnosis
Molluscum contagiosum is usually diagnosed based on a medical history and physical exam, with the lesions being unique and typically diagnosed on physical exam. The diagnosis is often made by a healthcare professional, such as a dermatologist, who will examine the skin and assess the appearance of the bumps to confirm the presence of molluscum contagiosum.
Molluscum contagiosum Treatment
Treatment options for molluscum contagiosum include various topical therapies like cantharidin, podophyllotoxin, salicylic acid, and imiquimod, which aim to induce an inflammatory reaction to accelerate recovery. Physical removal methods such as cryotherapy, curettage, and laser therapy are also utilized, although they may be associated with pain, local anesthesia, and the risk of scarring. In cases where treatment is necessary, individual lesion treatment is recommended, with YCANTH (cantharidin) being the only FDA-approved topical solution for molluscum contagiosum, administered by healthcare professionals.
Molluscum Contagiosum Epidemiology
As the market is derived using a patient-based model, the molluscum contagiosum epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total Molluscum Contagiosum Prevalence Cases, Molluscum Contagiosum Gender-Specific Cases in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
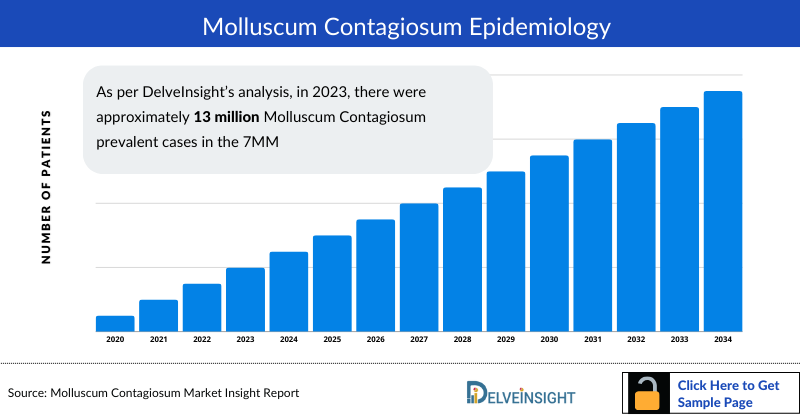
- As per DelveInsight’s analysis, in 2023, there were approximately 13 million Molluscum Contagiosum Prevalence Cases in the 7MM, out of which the US accounted for nearly 6 million cases. These cases are anticipated to rise during the forecasted period (2024-2034) primarily due to increased awareness among healthcare providers and the public regarding molluscum contagiosum that will enhance its identification and diagnosis.
- Among EU4 and the UK, France had the highest number of Molluscum Contagiosum Prevalent Cases, estimated to be nearly 1.3 million, followed by Germany with approximately 1.2 million cases in 2023.
- In 2023, in the US there were approximately 3 million cases of molluscum contagiosum in males and 4 million cases in females. The higher Molluscum Contagiosum prevalence of the disease among females in the US is attributed to factors such as differences in behavior, social interactions, and anatomical differences. For instance, females may have more frequent skin-to-skin contact or participate in activities that increase the risk of transmission. Additionally, hormonal factors or variations in immune responses between genders could also play a role in susceptibility to the infection.
- As per our analysis, in the UK there were approximately 518 thousand males and 633 thousand females affected with molluscum contagiosum in 2023. These cases are expected to change driven by the increased shifts in population demographics, lifestyle factors, and social behaviors in the UK.
- In 2023, Japan accounted for nearly 1.3 million cases of molluscum contagiosum. These cases are expected to increase by 2034 due to the high population density in Japan, which can facilitate the transmission of infectious diseases like molluscum contagiosum.
Molluscum Contagiosum Drugs Market Chapters
The drug chapter segment of the Molluscum Contagiosum Therapeutics Market Report encloses a detailed analysis of molluscum contagiosum-marketed drugs and late-stage (Phase III and Phase II) Molluscum Contagiosum Pipeline Drugs analysis. It also helps understand the Molluscum Contagiosum Clinical Trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Molluscum Contagiosum news and press releases.
Molluscum Contagiosum Marketed Drugs
- YCANTH (VP-102/cantharidin): Verrica Pharmaceuticals
In July 2023, the US FDA approved YCANTH (cantharidin) for the topical (used on the skin) treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older. It is administered to patients only by healthcare providers. Providers apply a single application of YCANTH on the areas of patients’ skin with molluscum bumps every 3 weeks as needed. YCANTH is a proprietary drug-device combination product that contains a GMP-controlled formulation of cantharidin delivered via a single-use applicator that allows for precise topical dosing and targeted administration for the treatment of molluscum. Furthermore, the active ingredient, cantharidin, is a naturally occurring vesicant that causes the degradation of desmosomal plaques, which helps treat topical dermatological conditions. Cantharidin is a potent and specific inhibitor of protein phosphatases 1 (PP1) and 2A (PP2A) that have keratolytic features inducing acantholysis.
Recently the Centers for Medicare & Medicaid Services (CMS) issued a permanent J-Code (J7354) for YCANTH, which is the only FDA-approved treatment for molluscum contagiosum. In addition, Verrica has completed a Phase II study of VP-102 for the treatment of common warts and a Phase II study of VP-102 for the treatment of external genital warts.
- ZELSUVMI (berdazimer) topical gel: Novan and Ligand Pharmaceuticals
In January 2024, the US FDA approved berdazimer gel, 10.3% (SB206) for the Molluscum Contagiosum Treatment in patients ages 1 year and older. This approval came just under a year after the FDA accepted its New Drug Application (NDA) for berdazimer in March of 2023.
SB206 is a combination product comprising a gel containing berdazimer sodium, which is a macromolecule featuring a polysiloxane backbone with N-diazeniumdiolate Nitric Oxide donors covalently attached. When co-administered with a hydrogel, this promotes the release of Nitric Oxide from berdazimer sodium upon application. Additionally, berdazimer sodium exhibits antiviral properties. Nitric oxide, a compound with diverse biological effects on the immune, cardio/pulmonary, and neurological systems, can exert either agonistic or antagonistic actions depending on its dosage and release kinetics. Developed through Novan's innovative NITRICIL macromolecular technology platform, this drug candidate is designed for the treatment of viral skin infections, with a specific focus on molluscum contagiosum.
Molluscum Contagiosum Emerging Drugs
- VBP-245 (povidone-iodine): Veloce Biopharma
VBP-245 is a topical gel under development by Veloce Biopharma to treat molluscum contagiosum. Povidone-iodine is a resistance-free, broad-spectrum antimicrobial agent that eradicates micro-organisms, including bacteria, viruses, yeasts, molds, fungi, and protozoa. The topical formulation has completed Phase II Molluscum Contagiosum Clinical Trials at three sites in the US for the treatment of molluscum contagiosum with no further update. However, the results have not yet disclosed by Veloce Biopharma.
Molluscum Contagiosum Drugs Market Insights
Molluscum Contagiosum treatment involves various drug classes, each with distinct mechanisms of action and therapeutic benefits. Topical immunomodulators like imiquimod stimulate the immune system, aiding in the clearance of lesions by enhancing the body's ability to recognize and eliminate the virus. Off-label use of topical antibiotics such as cidofovir or vidarabine targets viral replication directly, reducing viral load and facilitating lesion resolution. Nitric oxide-releasing agents exhibit antiviral effects against molluscum contagiosum, while cantharidin induces blistering upon topical application, aiding in lesion removal and skin healing. Additionally, antibiotics are prescribed to address secondary bacterial infections resulting from lesion irritation, minimizing complications.
Molluscum Contagiosum Market Outlook
Molluscum Contagiosum treatment aim to alleviate symptoms, accelerate lesion resolution, and reduce the risk of transmission. Topical medications may be prescribed to reduce lesion size, inflammation, and duration of infection. Common topical treatments include the use of podophyllotoxin solution or cream, imiquimod cream, and others to stimulate the body's immune response, aiding in the clearance of the virus.
Recently approved medications like ZELSUVMI (berdazimer) feature berdazimer sodium combined with a hydrogel. Similarly, YCANTH, a promising drug candidate, harnesses cantharidin, a compound applied to lesions to induce blistering, facilitating subsequent lesion removal. Furthermore, some off-label use of oral antiviral medications such as cimetidine or oral cidofovir has been reported in some cases. However, their efficacy remains uncertain, and they may be associated with potential side effects. Thus, the treatment landscape for molluscum contagiosum is expanding, driven by the demand for more effective and convenient therapies.
While traditional approaches like cryotherapy and curettage remain common, there's a growing interest in topical treatments, including immunomodulators, antiviral agents, and nitric oxide-based therapies. Additionally, research into novel treatment modalities such as photodynamic therapy and immune response modifiers is underway, offering potential future options. While there are various treatment options available for molluscum contagiosum, there are still several unmet needs in the management of this condition in terms of their safety and efficacy, patient convenience, pediatric formulations, and psychological impact.
While the pipeline for Molluscum Contagiosum Treatments is relatively limited, Veloce Biopharma's VBP-245, which utilizes povidone-iodine, shows potential for patients with this condition anticipated to positively influence the market size molluscum contagiosum during the forecast period [2024-2034].
According to DelveInsight, the overall Molluscum Contagiosum Market Dynamics are anticipated to change during the forecast period owing to the expected launch of emerging therapies, increasing awareness among healthcare providers and patients, coupled with technological advancements in diagnostic techniques, is expected to contribute to a growing pool of diagnosed cases.
- In 2023, the total Molluscum Contagiosum Treatment Market Size in the US accounted for nearly USD 1.2 billion in the US.
- Among the EU4 and the UK, France holds the highest Molluscum Contagiosum Treatment Market Size of around USD 168 million followed by Germany, and the UK with approximately USD 156 million, and USD 154 million respectively. These numbers are expected to change during the forecast period (2024-2034) driven by the ongoing research and development efforts that may lead to the discovery of more effective therapy for treating molluscum contagiosum.
Molluscum Contagiosum Drugs Uptake
This section focuses on the uptake rate of potential Molluscum Contagiosum drugs expected to be launched in the market during 2020–2034. For example, Veloce Biopharma’s VBP-245 is a topical gel that is a resistance-free, broad-spectrum antimicrobial agent that eradicates micro-organisms, including bacteria, viruses, yeasts, molds, fungi, and protozoa.
Molluscum Contagiosum Pipeline Development Activities
The Molluscum Contagiosum Therapeutics Market Report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Molluscum Contagiosum Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Molluscum Contagiosum Therapeutics Market Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Molluscum Contagiosum emerging therapies.
KOL Views
To keep up with current Molluscum Contagiosum Market Trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving molluscum contagiosum treatment market landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Feinberg School of Medicine, Saint Louis University, University of California, University Medical Center Schleswig Holstein, Kiel, Institut de la Vision, Sapienza University of Rome, and University of Birmingham, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or molluscum contagiosum market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Region: KOL Views
The US The end goal of any approach is the destruction of the lesion to halt further spread of the disease and auto-inoculation, which is why most of the treatments are mechanical to traumatize the lesions. Recently, several antiviral and immune-modulating options have been added to our therapeutic armamentarium.
Germany We found no strong evidence either for or against the most commonly used treatment options for molluscum contagiosum. Allowing for the natural resolution of the infection remains a reasonable approach. We found some evidence to suggest that 10% potassium hydroxide is more effective than saline; a 5% solution of potassium hydroxide is favored compared to a 2.5% solution of potassium hydroxide; 10% povidone-iodine solution plus 50% salicylic acid plaster is favored compared to salicylic acid plaster alone; and homeopathic calcarea carbonica is favored compared to placebo.
The UK Most over-the-counter (OTC) products for molluscum contagiosum (MC) do not include sufficient information about their plant-based ingredients or appropriate dosing, according to an analysis of eight such products available to U.S. consumers. It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them.
Physician’s View
According to physicians, a primary challenge in molluscum contagiosum is the lack of consensus on optimal treatment protocols for managing molluscum contagiosum. They often express frustration over the absence of standardized guidelines, leading to variability in treatment approaches and outcomes. Additionally, the limited efficacy of available treatments and the potential for adverse effects pose further challenges in providing effective care for patients with molluscum contagiosum.
Qualitative Analysis
We perform Qualitative and Molluscum Contagiosum Drugs Market Intelligence analysis using various approaches, such as SWOT analysis and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Attribute Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in Molluscum Contagiosum trials, one of the most important primary outcome measures is complete eschar removal.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Molluscum Contagiosum Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The reimbursement challenges related to medical care and treatment for individuals with molluscum contagiosum can be significant as they often require specialized medical attention, covering the costs of diagnosis, treatment, and ongoing care.
Health insurance plans may not fully cover limited coverage of some medical treatments, and therapies specific to molluscum contagiosum. This can result in high out-of-pocket expenses for families seeking the best care for their loved ones. Moreover, it requires specialized care from healthcare providers with expertise. Finding and accessing such specialists may be challenging, and the associated costs may not always be fully reimbursed by insurance.
Molluscum Contagiosum Therapeutics Market Report Scope
- The Molluscum Contagiosum therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and Molluscum Contagiosum emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current Molluscum Contagiosum Treatment Market Landscape.
- A detailed review of the molluscum contagiosum treatment market, historical and forecasted Molluscum Contagiosum treatment market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Molluscum Contagiosum treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM molluscum contagiosum drugs market.
Molluscum Contagiosum Therapeutics Market Report Insights
- Patient-based Molluscum Contagiosum Market Forecasting
- Therapeutic Approaches
- Molluscum Contagiosum Pipeline Drugs Analysis
- Molluscum Contagiosum Market Size and Trends
- Existing and Future Molluscum Contagiosum Therapeutics Market Opportunity
Molluscum Contagiosum Therapeutics Market Report Key Strengths
- 11 years Molluscum Contagiosum Market Forecast
- The 7MM Coverage
- Molluscum Contagiosum Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Molluscum Contagiosum Drugs Uptake
- Key Molluscum Contagiosum Market Forecast Assumptions
Molluscum Contagiosum Therapeutics Market Report Assessment
- Current Molluscum Contagiosum Treatment Market Practices
- Molluscum Contagiosum Unmet Needs
- Molluscum Contagiosum Pipeline Drugs Profiles
- Molluscum Contagiosum Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Molluscum Contagiosum Therapeutics Market Insights
- What was the molluscum contagiosum drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What was the total molluscum contagiosum market size by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will YCANTH, and ZELSUVMI affect the treatment paradigm of molluscum contagiosum?
- How will VBP-245 (povidone-iodine) compete with other approved products?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Molluscum Contagiosum Epidemiology Insights
- What are the disease risks, burdens, and Molluscum Contagiosum unmet needs? What will be the growth opportunities across the 7MM concerning the patient population with molluscum contagiosum?
- What is the historical and forecasted molluscum contagiosum patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the above-mentioned countries, which country would have the highest diagnosed prevalent molluscum contagiosum population during the forecast period (2024–2034)?
- What factors are factors contributing to the growth of molluscum contagiosum cases?
Current Molluscum Contagiosum Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the molluscum contagiosum treatment? What are the current guidelines for treating molluscum contagiosum in the US and Europe?
- How many companies are developing therapies for the molluscum contagiosum treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating molluscum contagiosum?
- What are the recent novel therapies, targets, Molluscum Contagiosum mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current Molluscum Contagiosum treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of approved therapies?
- What is the 7MM historical and forecasted Molluscum Contagiosum Market?
Reasons to Buy
- The Molluscum Contagiosum Therapeutics Market Report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Molluscum Contagiosum Drugs Market.
- Insights on patient burden/disease Molluscum Contagiosum Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Molluscum Contagiosum Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the Molluscum Contagiosum Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies for molluscum contagiosum, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Molluscum Contagiosum Therapeutics Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles