Myelodysplastic Syndrome Market Summary
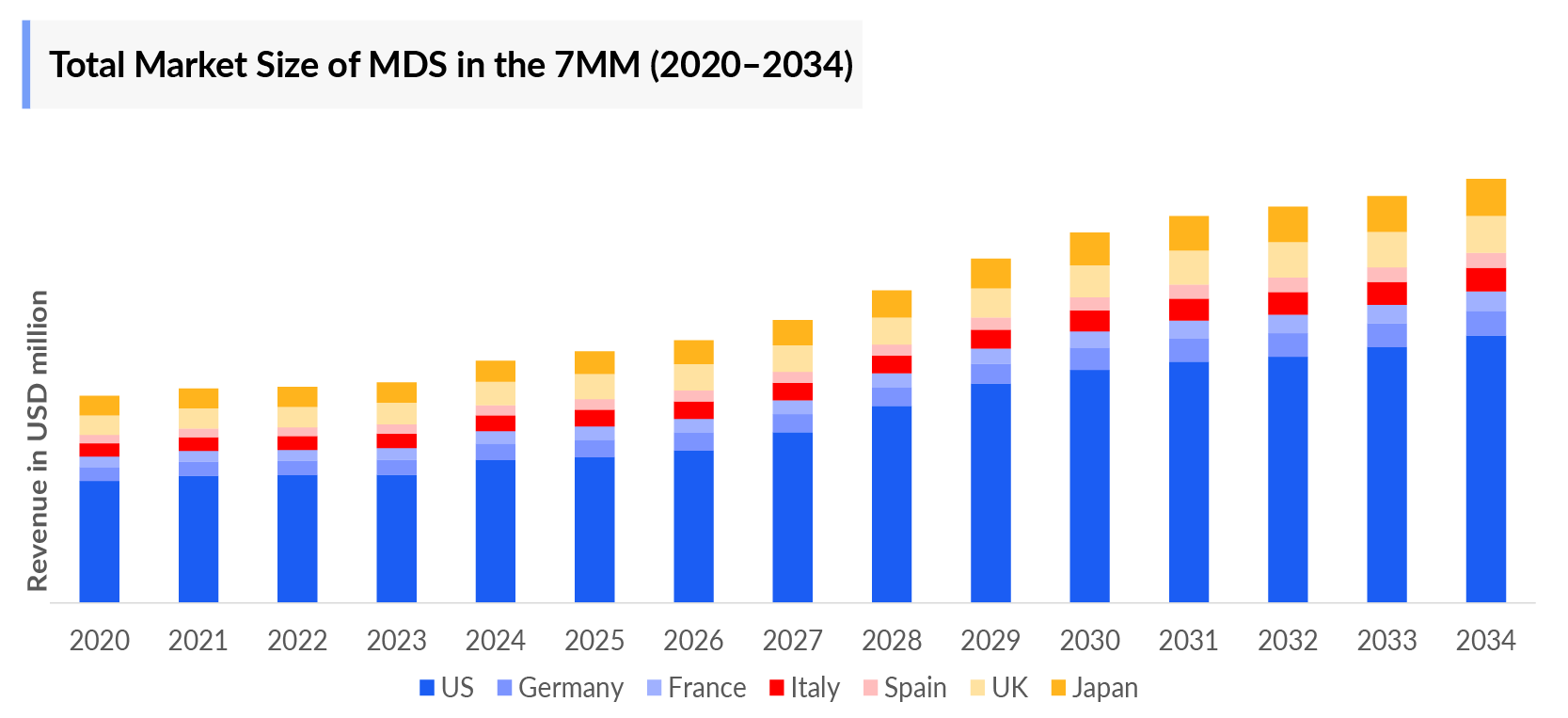
- The total Myelodysplastic Syndrome Market Size in 2023 was more than USD 2,800 Million in the 7MM, and the largest market size was occupied by the US.
- Among EU4 and the UK, Germany will capture the maximum revenue share, followed by France in 2034.
- The Myelodysplastic Syndrome Comapnies developing therapies include - Bristol-Myers Squibb, Astex Pharmaceutical, Taiho Oncology, Fibrogen, AbbVie, Gilead SciencesNovartis, Syros Pharmaceuticals, Takeda, Pfizer, Geron Corporation, Karyopharm Therapeutics, Antengene Corporation, BerGenBio ASA, Jazz Pharmaceuticals, Aprea Therapeutics, Sanofi, Medac, and others.
Myelodysplastic Syndrome Market & Epidemiology Insights
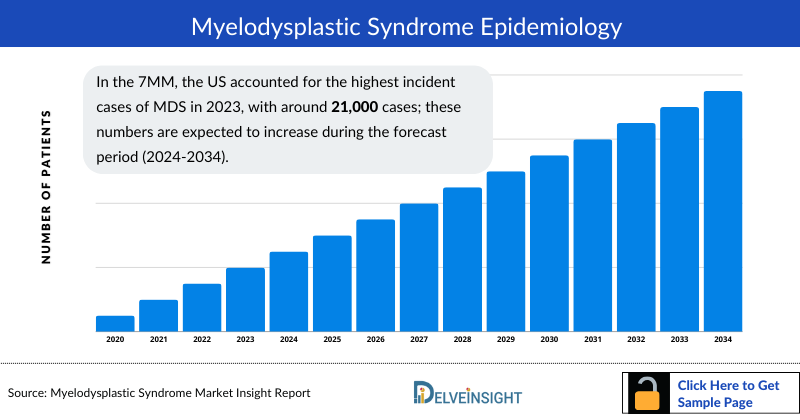
- In the 7MM, the US accounted for the highest incident cases of MDS in 2023, with around 21,000 cases; these numbers are expected to increase during the forecast period (2024-2034).
- Among the risk-specific cases, low-risk cases accounted for the highest number of incident cases in the US in 2023.
- Among the mutation-specific cases, SF3B1 accounted for the maximum incident cases, followed by TET2, with nearly 25% and 20% cases respectively in the US in 2023.
- In April 2024, the European Commission expanded approval of Bristol Myers Squibb's REBLOZYL (luspatercept) to include first-line treatment of transfusion-dependent anemia in adults with lower-risk myelodysplastic syndrome (LR-MDS).
- In February 2024, Gilead Sciences announced that it has discontinued the Phase III ENHANCE-3 study of magrolimab in AML and that the US FDA placed all magrolimab studies in myelodysplastic syndromes and AML, including related expanded access programs, on full clinical hold.
- In January 2024, Novartis announced that it is discontinuing sabatolimab after the failure to prioritize other key programs in its portfolio. The Phase III Stimulus trial (NCT04266301) investigated the drug in combination with the chemotherapy drug azacitidine in patients with high or very high-risk myelodysplastic syndrome.
Request a sample to unlock the CAGR for "Myelodysplastic Syndrome Market Forecast"
Key Factors Driving Myelodysplastic Syndrome Market:
- Rising disease prevalence: An aging global population is increasing the incidence of myelodysplastic syndromes (MDS), as the disease predominantly affects older adults.
- Advancements in therapeutics: Ongoing R&D and the introduction of novel agents, including hypomethylating agents, targeted therapies, and combination regimens, are improving treatment outcomes and expanding the market.
- Improved diagnosis and awareness: Greater clinical awareness, enhanced diagnostic tools, and broader use of genetic and molecular testing are enabling earlier and more accurate diagnosis of MDS.
- Growing unmet medical need: Limited curative options beyond stem cell transplantation are driving demand for effective, disease-modifying therapies and long-term management solutions.
- Supportive regulatory and reimbursement landscape: Regulatory incentives for rare diseases and improved reimbursement policies in key markets are encouraging pharmaceutical investment and accelerating drug development.
DelveInsight’s "Myelodysplastic Syndrome Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of the Myelodysplastic Syndrome historical and forecasted epidemiology as well as the Myelodysplastic Syndrome therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Myelodysplastic Syndrome Drug Market report provides current treatment practices, emerging drugs, Myelodysplastic Syndrome market share of individual therapies, and current and forecasted Myelodysplastic Syndrome market size from 2020 to 2034, segmented by seven major markets. The report also covers current MDS treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Myelodysplastic Syndrome Market |
|
|
Myelodysplastic Syndrome Market Size | |
|
Myelodysplastic Syndrome Companies |
Bristol-Myers Squibb, Astex Pharmaceutical, Taiho Oncology, Fibrogen, AbbVie, Gilead SciencesNovartis, Syros Pharmaceuticals, Takeda, Pfizer, Geron Corporation, Karyopharm Therapeutics, Antengene Corporation, BerGenBio ASA, Jazz Pharmaceuticals, Aprea Therapeutics, Sanofi, Medac, and others |
|
Myelodysplastic Syndrome Epidemiology Segmentation |
|
Myelodysplastic Syndrome Disease Understanding
Myelodysplastic syndrome is a heterogeneous group of hematologic neoplasms classically described as a clonal disorder of hematopoietic stem cells leading to dysplasia and ineffective hematopoiesis in the bone marrow. In Myelodysplastic Syndrome, also known as myelodysplasia, the bone marrow cells do not develop into mature blood cells; instead, these cells stay within the bone marrow in an immature state. There are many subtypes of Myelodysplastic Syndrome; some cases are mild, while others are more severe and carry a high risk of becoming acute myelogenous leukemia. The main feature of MDS is that they cause low blood cell counts. Sometimes this is found on blood tests, even before symptoms appear. In other cases, symptoms related to shortages of one or more types of blood cells (cytopenias) are the first signs of Myelodysplastic Syndrome.
Myelodysplastic Syndrome Diagnosis
Diagnosis typically involves a thorough clinical evaluation, including a detailed medical history, physical examination, and laboratory tests such as complete blood count (CBC), peripheral blood smear, and bone marrow aspiration and biopsy. In CBC, characteristic findings include cytopenias, particularly anemia, neutropenia, and thrombocytopenia, which may prompt further investigation. Peripheral blood smear often reveals dysplastic changes in blood cells, such as abnormal morphology or cytoplasmic vacuolization. Bone marrow examination is crucial for definitive diagnosis, where dysplastic changes in the bone marrow cells, including abnormal cell morphology, increased blasts, and dysplastic megakaryocytes, confirm the presence of MDS. Overall, a comprehensive diagnostic approach is necessary to accurately identify MDS, which guides appropriate management and prognostication for affected individuals.
Further details related to diagnosis will be provided in the report…
Myelodysplastic Syndrome Treatment
The standard care of treatment for MDS includes supportive care, drug therapy, and stem cell transplantation. MDS patients with symptoms caused by low blood counts are given supportive care to relieve symptoms and improve their quality of life. While drug therapy may slow the disease’s progression, certain patients can be cured with aggressive treatment with chemotherapy followed by stem cell transplant using stem cells from a donor. Erythropoietin stimulating agents are considered the first-line treatment of low-risk MDS patients displaying pretreatment variables predictive of response to treatment.
Further details related to treatment will be provided in the report…
Myelodysplastic Syndrome Epidemiology
The Myelodysplastic Syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the Total Incident Population of Myelodysplastic Syndrome, Age-specific Incident Population of Myelodysplastic Syndrome, Subtype-specific Incident Population of Myelodysplastic Syndrome, Risk-specific Incident Population of Myelodysplastic Syndrome, and Mutation-specific Incident Population of Myelodysplastic Syndrome in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In the 7MM, the US accounted for the highest Myelodysplastic Syndrome incident cases in 2023, with around 21,000 cases; these numbers are expected to increase during the forecast period.
- Among the age-specific cases, the cases from the age group 80+ accounted for the highest incident cases in 2023 in the US, with nearly 40% of total cases.
- Among the subtype-specific cases, RAEB/MDS-EB accounted for the highest number in the US in 2023.
- Amongst EU4 and the UK, Germany accounted for the highest incident cases, followed by France, while Spain occupied the bottom of the ladder in 2023.
Myelodysplastic Syndrome Epidemiology Segmentation
- Total Incident Population of Myelodysplastic Syndrome
- Age-specific Incident Population of Myelodysplastic Syndrome
- Subtype-specific Incident Population of Myelodysplastic Syndrome
- Risk-specific Incident Population of Myelodysplastic Syndrome
- Mutation-specific Incident Population of Myelodysplastic Syndrome
Recent Developments In The Myelodysplastic Syndrome Treatment Landscape
- In September 2025, Lupin received FDA approval for its generic Lenalidomide Capsules (2.5–25 mg), a treatment for multiple myeloma and certain cases of myelodysplastic syndromes (MDS). The product, bioequivalent to Bristol-Myers Squibb’s Revlimid®, will be manufactured at Lupin’s Pithampur facility in India.
- In September 2025, Minovia Therapeutics Ltd. announced that the U.S. FDA granted Fast Track Designation to its lead investigational drug, MNV-201, for treating Myelodysplastic Syndrome (MDS), a serious age-related blood disorder. This designation complements existing Fast Track and Rare Pediatric Disease approvals for MNV-201 in treating Pearson Syndrome, a rare and life-threatening mitochondrial disorder currently being studied in a Phase 2 trial.
- In March 2025, Faron Pharmaceuticals Ltd. a biopharmaceutical company specializing in immune system manipulation for cancer treatment, announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation for bexmarilimab, its leading drug candidate for myelodysplastic syndromes (MDS).
- In January 22, 2025, Medexus announced that the FDA approved GRAFAPEX™, an alkylating agent, with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation (alloHSCT) in adult and pediatric patients aged one year and older with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
- In January 2025, R289, a potent and selective dual inhibitor of IRAK1 and IRAK4, received orphan drug designation from the FDA for the treatment of patients with myelodysplastic syndromes (MDS). An ongoing open-label phase 1b study is evaluating R289’s safety, tolerability, pharmacokinetics, and preliminary activity, specifically in patients with LR-MDS who are relapsed or refractory to prior therapies.
- In January 2025, Rigel Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug designation to R289 for the treatment of myelodysplastic syndromes (MDS).
- In December 2024, Rigel Pharmaceuticals, Inc. announced that the FDA granted Fast Track designation to R289 for the treatment of patients with previously treated transfusion-dependent lower-risk myelodysplastic syndrome (LR-MDS).
- In November 2024, Marks Shorla Oncology announced that the FDA approved imatinib (Imkeldi), making it the first oral, liquid tyrosine kinase inhibitor (TKI) approved for treating various cancers, including gastrointestinal tumors (GIST), myelodysplastic syndromes (MDS), myeloproliferative disease (MPD), chronic myeloid leukemia (CML), and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
- In September 11, 2024, Agios Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation to its novel pyruvate kinase (PK) activator, tebapivat (AG-946), for the treatment of myelodysplastic syndromes (MDS).
Myelodysplastic Syndrome Drug Analysis
The drug chapter segment of the Myelodysplastic Syndrome market forecast report encloses a detailed analysis of the marketed late-stage (Phase III), and mid-stage (Phase II) Myelodysplastic Syndrome pipeline drugs. The Myelodysplastic Syndrome marketed drugs segment encloses drugs such as VIDAZA (Bristol Myers Squibb), DACOGEN (Johnson & Johnson), REBLOZYL (Bristol Myers Squibb), and others. Furthermore, the current key players for emerging drugs and their respective drug candidates include Abbvie (Venetoclax), Syros Pharmaceuticals (Tamibarotene), and others. The drug chapter also helps understand the Myelodysplastic Syndrome clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest Myelodysplastic Syndrome news and press releases.
Myelodysplastic Syndrome Marketed Drugs
-
VIDAZA (azacitidine): Bristol Myers Squibb
VIDAZA is a pyrimidine nucleoside analog of cytidine. VIDAZA is believed to exert its antineoplastic effects by causing hypomethylation of DNA and direct cytotoxicity on abnormal hematopoietic cells in the bone marrow. The concentration of azacitidine required for maximum inhibition of DNA methylation in vitro does not cause major suppression of DNA synthesis. It was first approved by the US FDA in May 2004 for the Myelodysplastic Syndrome treatment.
-
DACOGEN (decitabine): Johnson & Johnson
DACOGEN is a nucleoside metabolic inhibitor indicated for the treatment of adult patients with myelodysplastic syndromes including previously treated and untreated, de novo and secondary MDS. Decitabine-induced hypomethylation in neoplastic cells may restore normal function to genes that are critical for the control of cellular differentiation and proliferation. In rapidly dividing cells, the cytotoxicity of decitabine may also be attributed to the formation of covalent adducts between DNA methyltransferase and decitabine incorporated into DNA. Non-proliferating cells are relatively insensitive to decitabine. It was first approved by the US FDA in May 2006.
Comparison of Myelodysplastic Syndrome Marketed Drugs | ||||
|
Product |
Company |
MoA |
RoA |
Approval Year (US) |
|
VIDAZA |
Bristol Myers Squibb |
Hypomethylation of DNA |
SC |
2004 |
|
DACOGEN |
Johnson & Johnson |
Inhibition of DNA methyltransferase |
IV infusion |
2006 |
|
INQOVI |
Astex Pharmaceutical/Taiho Oncology |
nucleoside metabolic inhibitor / cedazuridine cytidine deaminase inhibitor |
Oral |
2020 |
|
REBLOZYL |
Bristol Myers Squibb |
TGF-beta protein inhibitor |
SC injection |
2020 |
Myelodysplastic Syndrome Emerging Drugs
-
Venetoclax: Abbvie
-
Tamibarotene: Syros Pharmaceuticals
Comparison of Myelodysplastic Syndrome Emerging Therapies | |||||
|
Emerging Drug |
Company |
Phase |
Molecule Type |
MoA |
RoA |
|
Venetoclax |
Abbvie |
III |
Small molecule |
BCL-2 inhibitor |
Oral |
|
Tamibarotene |
Syros Pharmaceuticals |
III |
Small molecule |
RAA inhibitor |
Oral |
|
Briquilimab |
Jasper Therapeutics |
I |
Monoclonal antibody |
Proto-oncogene protein c-kit inhibitors |
IV |
Myelodysplastic Syndrome Drug Class Insight
Myelodysplastic Syndrome Market Outlook
Myelodysplastic Syndrome Market Key Findings
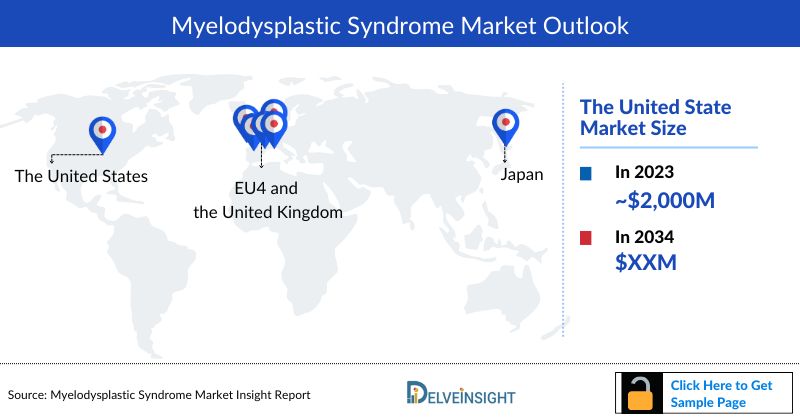
- In the 7MM, the US accounted for the largest Myelodysplastic Syndrome market size in 2023.
- The total Myelodysplastic Syndrome Market Size in the US was estimated to be nearly USD 2,000 million in 2023, which is expected to grow during the forecast period (2024–2034).
- Among the second-line therapies, REBLOZYL accounted for the largest Myelodysplastic Syndrome market size in the US in 2023.
- Among EU4 and the UK, Germany accounted for the largest Myelodysplastic Syndrome market size, followed by France in 2023.
Myelodysplastic Syndrome Drugs Uptake
Myelodysplastic Syndrome Pipeline Development Activities
KOL- Views on Myelodysplastic Syndrome Market Report
|
KOL Views |
|
“When misdiagnoses occur, population estimates in disease. – in registries such as SEER or cancer registries – can miss the mark, and either overestimate or underestimate population incidence rates and prevalence. This, in turn, affects the allocation of appropriate resources to treat conditions such as MDS, and survival estimates. In the case of MDS, we believe these population registries underestimate both the incidence and prevalence of MDS and the impact of the disease on healthcare resource use. For example, patients with MDS, who tend to have anemia or low platelet counts, use a disproportionate amount of blood products.” |
|
“For patients with lower-risk MDS, clinical treatment focuses on the management of symptoms resulting from peripheral blood cytopenias and minimizing the need for transfusions, with a wide array of treatment options available ranging from erythropoiesis-stimulating agents to immunosuppression, lenalidomide, HMA, and TGF-β pathway inhibitors.” |
Myelodysplastic Syndrome Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Myelodysplastic Syndrome Market Access and Reimbursement
The treatment and management of Myelodysplastic Syndrome are expensive. The BMS Access Support program is dedicated to helping patients access their prescribed BMS medications. It offers benefit investigations, prior authorization assistance, appeals process support, and information on financial support options. The BMS Access Support Co-Pay Assistance Program assists with out-of-pocket co-payment or co-insurance requirements for eligible, commercially insured patients who have been prescribed certain BMS products, including REBLOZYL.
Detailed market access and reimbursement assessment will be provided in the final report...
Scope of the Myelodysplastic Syndrome Market Forecast Report
- The Myelodysplastic Syndrome market forecast report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Myelodysplastic Syndrome treatment landscape.
- A detailed review of the Myelodysplastic Syndrome market, historical and forecasted Myelodysplastic Syndrome treatment market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Myelodysplastic Syndrome market forecast report provides an edge while developing business strategies by understanding trends through SWOT analysis and KOL views, patient journey, and treatment preferences that help shape and drive Myelodysplastic Syndrome.
Myelodysplastic Syndrome Market Forecast Report Insights
- Patient-based Myelodysplastic Syndrome Market Forecast
- Myelodysplastic Syndrome Therapeutic Approaches
- Myelodysplastic Syndrome Pipeline Analysis
- Myelodysplastic Syndrome Market Size
- Myelodysplastic Syndrome Market Trends
- Existing and Future Myelodysplastic Syndrome Market Opportunity
Myelodysplastic Syndrome Market ForecastReport Key Strengths
- 11 Years Myelodysplastic Syndrome Market Forecast
- The 7MM Coverage
- Myelodysplastic Syndrome Epidemiology Segmentation
- Key Cross Competition
- Myelodysplastic Syndrome Drugs Uptake
- Key Myelodysplastic Syndrome Market Forecast Assumptions
Myelodysplastic Syndrome Treatment Market Size Report Assessment
- Current Myelodysplastic Syndrome Treatment Market Size Practices
- Myelodysplastic Syndrome Unmet Needs
- Myelodysplastic Syndrome Pipeline Product Profiles
- Myelodysplastic Syndrome Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Myelodysplastic Syndrome Market Drivers
- Myelodysplastic Syndrome Market Barriers
FAQs Regarding Myelodysplastic Syndrome Market Report:
- What was the Myelodysplastic Syndrome market size, the Myelodysplastic Syndrome treatment market size by therapies, market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for Myelodysplastic Syndrome?
- What are the disease risks, burdens, and Myelodysplastic Syndrome unmet needs? What will be the growth opportunities across the 7MM concerning the patient population with Myelodysplastic Syndrome?
- What are the current options for the Myelodysplastic Syndrome treatment? What are the current guidelines for treating Myelodysplastic Syndrome in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in Myelodysplastic Syndrome?
Reasons to Buy Myelodysplastic Syndrome Market Forecast Report
- The patient-based Myelodysplastic Syndrome market forecasting report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Myelodysplastic Syndrome drug market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the Myelodysplastic Syndrome drug market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of current therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Access Exclusive Data Now! Click here to Read More about the Related Articles @ Latest DelveInsight Blog

-03.png)
-01.png)