Neuroendocrine Tumors Market
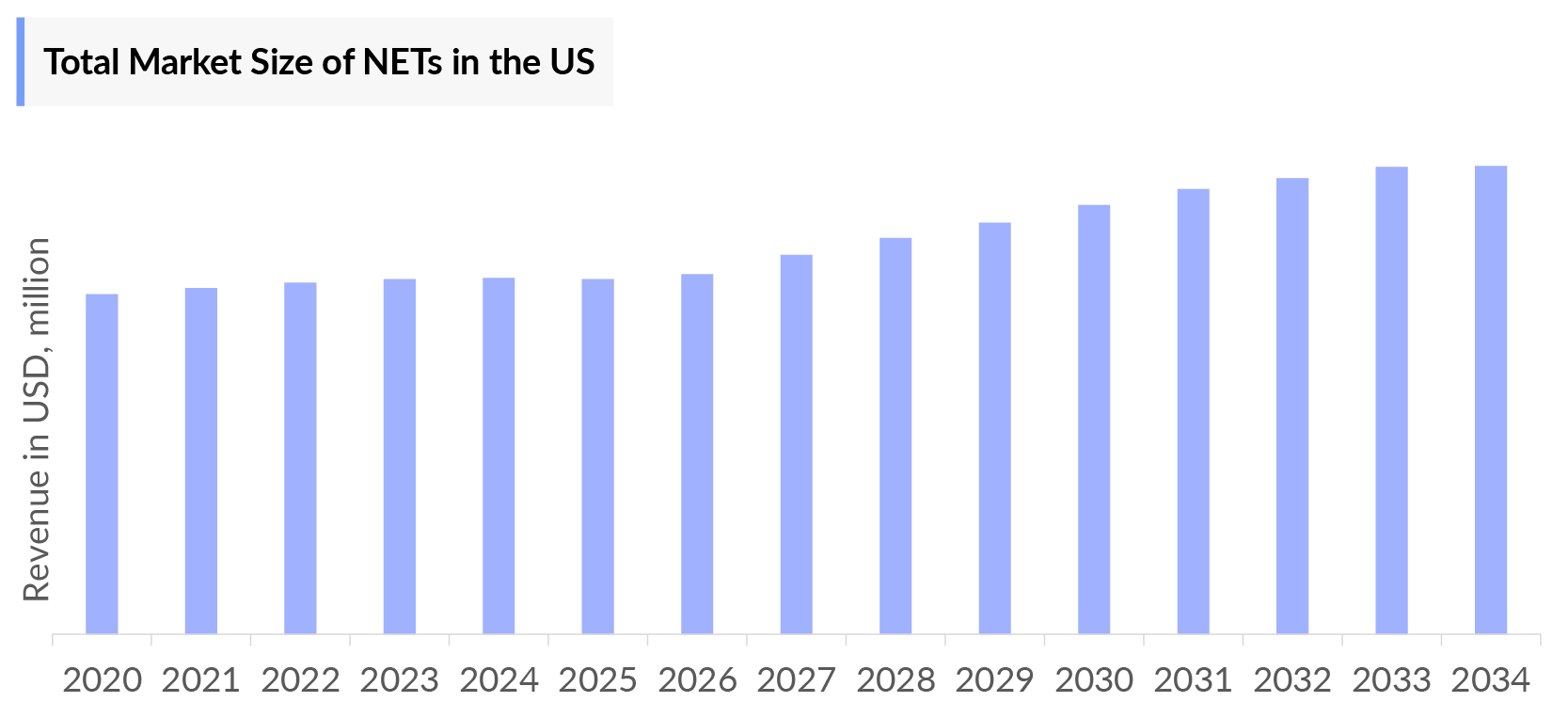
- The total Neuroendocrine Tumors Market Size in the US was estimated to be nearly USD 1,530 million in 2023, which is expected to grow with a significant CAGR during the forecast period.
- In recent years, radioactive isotopes have garnered significant attention from the pharmaceutical industry due to their growing clinical use in both diagnosing and treating various cancers.
- The management of Neuroendocrine Tumors is increasingly shifting towards endoscopic approaches, reducing the need for surgery. Many Neuroendocrine Tumors are detected during routine endoscopies or colonoscopies and are simultaneously removed if they are small enough.
- Long-acting octreotide formulations, such as Novartis' SANDOSTATIN LAR, have been widely used as a first-line treatment for Neuroendocrine Tumors. Despite its strong market uptake post-approval, SANDOSTATIN LAR's sales are now declining due to generic competition in the European market. However, no generic version has been launched yet in the US because of its complex formulation. Teva Pharmaceuticals attempted to develop a generic but was unsuccessful, leaving the market open for competition to see which company would successfully introduce a generic alternative.
- Since only beta-emitting radioisotopes are currently approved, companies are now focusing on developing alpha emitters. Alpha particles have a higher linear energy transfer (LET) than beta particles, allowing for greater biological damage and the potential to overcome radio- and chemoresistance. Neuroendocrine Tumors Companies like Bristol Myers Squibb (BMS)/RayzeBio, Radiomedix/Orano Med, Perspective Therapeutics, and others are actively working in this area.
- The Neuroendocrine Tumors indication has a strong pipeline, with Neuroendocrine Tumors companies actively developing Neuroendocrine Tumors therapies. Key Neuroendocrine Tumors Companies such as ITM Solucin GmbH, Camurus, Ipsen, Takeda, Exelixi, BMS, RayzeBio, Radiomedix, OranoMed, Crinetics Pharmaceuticals, Chimerix, Perspective Therapeutics, Teclison, Chimeric Therapeutics, PharmaMar, Incyte Corporation, Enterome, Elicera Therapeutics and others.
Request for Unlocking the Sample Page of the "Neuroendocrine Tumors Treatment Market"
DelveInsight’s “Neuroendocrine Tumors Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Neuroendocrine Tumors, historical and forecasted epidemiology as well as Neuroendocrine Tumors market trends in the United States and EU4 (Germany, France, Italy, and Spain) and the United Kingdom.
The Neuroendocrine Tumors Treatment Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted Neuroendocrine Tumors market size from 2020 to 2034. The report also covers current Neuroendocrine Tumors treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Neuroendocrine Tumors Treatment Market: Understanding and Algorithm
Neuroendocrine Tumors can start in different parts of the body. Like all cancers, Neuroendocrine Tumors are named after the place they start growing. For example, a Neuroendocrine Tumors that starts in the lung is called a lung NET. This is the primary cancer. If the cancer spreads to another part of the body, it’s called secondary cancer. The maximum number of times, Neuroendocrine Tumors start from the digestive system, affecting the stomach, small bowel, large bowel, pancreas, and rectum. The least number of Neuroendocrine Tumors start from the lungs.
The Neuroendocrine Tumors can also start from the esophagus, appendix, skin, prostate, womb, adrenal, and other body parts. There are ways of classifying Neuroendocrine Tumors. They can be classified as functional as well as nonfunctional. They can be indolent or aggressive. WHO has given a separate classification of grouping Neuroendocrine Tumors. The exact causes of Neuroendocrine Tumors are unknown, but there are numerous associated risk factors, including pituitary adenoma, Von Hippel-Lindau (VHL), pheochromocytomas, etc. Neuroendocrine Tumors grow at different rates, but they often grow very slowly. Some might not grow at all for months or years. So, it might not need treatment straight away.
Neuroendocrine Tumors Diagnosis
Various tests are used, including urine test, CT scan, ultrasound, MRI, PET-CT scan, radioactive scan, colonoscopy, endoscopy, biopsy, and other blood tests to diagnose Neuroendocrine Tumors. The diagnostic tests that health care experts use are determined by several factors, including the type of Neuroendocrine Tumors suspected, the signs and symptoms expected, age and general health, and the results of previous medical tests. Testing can help the doctor understand the specifics of the tumor and develop a treatment plan for the neuroendocrine cancer. The tests recommended are as follows:
Imaging tests
- Computed tomography (CT)
- Magnetic Resonance Imaging (MRI)
- Positron Emission Tomography (PET)
- Somatostatin Receptor Scintigraphy (SRS)
- Gallium 68 (Ga-68) or Copper-64 (Cu-64) scan
- Octreoscan
- FDG (Fluorodeoxyglucose) Scan
- MIBG (Meta-iodobenzylguanidine) scan
- Endoscopy
- Colonoscopy
- Upper GI Endoscopy
- Bronchoscopy
Tissue testing
- Endoscopic Ultrasound-Guided Fine-Needle Aspirations (EUS-FNA)
- Biopsies For lung Fine-Needle Aspiration (FNA)
- Laboratory tests for tumor tissue samples Immunohistochemistry (IHC)
5-HIAA tests
Blood tests
- Chromogranin A (CGA)
- Blood chemistry tests
- NETest
Barriers to diagnosis and community recommendations
Further details related to diagnosis are provided in the report…
Neuroendocrine Tumors Treatment
Treatment for an Neuroendocrine Tumors depends on several things, including where the cancer is, its size, and whether it has spread. Surgery has been traditionally considered to be the main treatment of NET; however, in recent decades there has been a considerable evolution of several nonsurgical treatments that have expanded the therapeutic options of these neoplasms. In neuroendocrine neoplasm (NENs) G1 or G2, surgery to cure can be considered even in the presence of liver or lymph node metastases. In patients with advanced disease, tumor debulking techniques such as hepatic artery embolization (HAE), selective internal radiotherapy (SIRT), radiofrequency ablation (RFA), and palliative hepatic cytoreductive surgery may significantly decrease the tumor burden or lead to symptomatic improvement of hormone excess states. As the majority of NENs express Somatostatin receptors (SSTRs), long-acting somatostatin analogs (SSAs) play an important role in the treatment of patients with NENs and may result in symptomatic, biochemical, and objective responses.
Neuroendocrine Tumors Epidemiology
The Neuroendocrine Tumors epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Neuroendocrine Tumors, Cases of Neuroendocrine Tumors by Grade, Stage-specific Cases of Neuroendocrine Tumors, Cases of Neuroendocrine Tumors by Site, and Cases of Neuroendocrine Tumors by Functional Status covering the United States and EU4 (Germany, France, Italy, and Spain) and United Kingdom from 2020 to 2034.
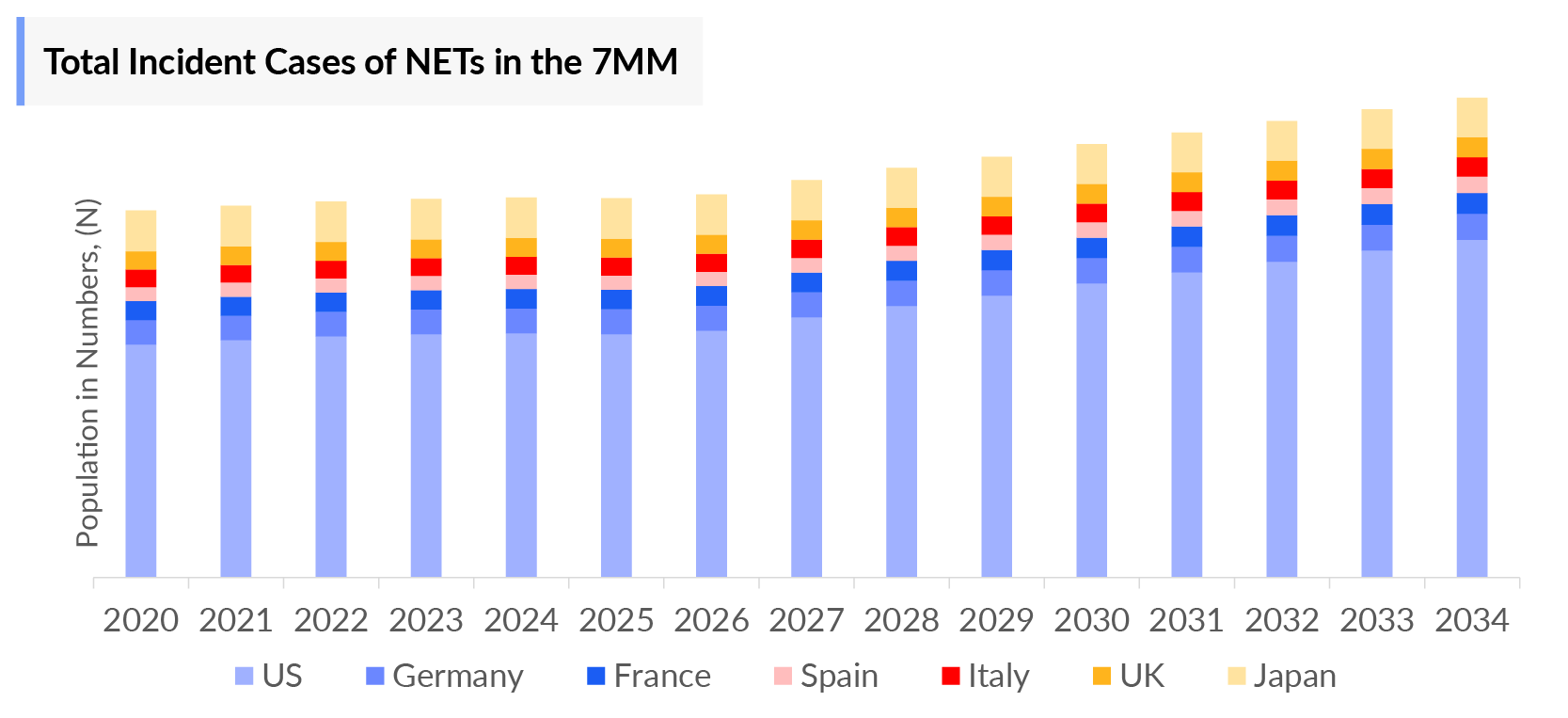
- The total number of Neuroendocrine Tumors incident cases in the US was nearly 29,500 cases in 2023 and is projected to increase during the forecasted period.
- Non-functional/Asymptomatic/Unknown Neuroendocrine Tumors hold the largest contributors to the overall Neuroendocrine Tumors by functional status. The incident cases of Neuroendocrine Tumors in the United States were nearly 16,000 for Non-functional/Asymptomatic/Unknown Neuroendocrine Tumors and nearly 15,000 for Functional Neuroendocrine Tumors in 2023.
- Among EU4 and the UK, the highest number of cases for Neuroendocrine Tumors was found in the UK which was estimated to be nearly 30% of cases in EU4 and the UK in 2023.
- The establishment of comprehensive cancer registries and better reporting systems are now providing more accurate data on the incidence of Neuroendocrine Tumors. This is helping in understanding the true incidence of these tumors.
- Approximately 35–37% of patients had low-grade neuroendocrine neoplasms. This was followed by intermediate-grade neoplasms in 17–24% of patients, while only 6–7% of patients had high-grade disease. The grade status of the majority of patients remains unknown.
Neuroendocrine Tumors Drugs Market Chapters
The drug chapter segment of the Neuroendocrine Tumors treatment market report encloses a detailed analysis of the marketed and the late, mid, and early stage (Phase III, Phase II, and Phase I/II) Neuroendocrine Tumors pipeline drugs analysis. The marketed drugs segment encloses drugs such as WELIREG (belzutifan/MK-6482), LUTATHERA (lutetium Lu 177 dotatate), SUTENT (sunitinib malate), AFINITOR (everolimus), SOMATULINE DEPOT (lanreotide), DEMSER Capsule (metyrosine), and AZEDRA (iobenguane I 131; Ultratrace iobenguane I-131; Raiatt MIBG-I 131 Injection). The drug chapter also helps understand the Neuroendocrine Tumors clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest Neuroendocrine Tumors news and press releases.
Neuroendocrine Tumors Marketed Drugs
- LUTATHERA (lutetium Lu 177 dotatate): Advanced Accelerator Applications (AAA)/Novartis
A 177Lu-labeled somatostatin analog peptide, Lutetium Lu 177 dotatate (177Lu-DOTATATE; LUTATHERA), belongs to an emerging form of treatment called Peptide Receptor Radionuclide Therapy (PRRT), which involves targeting tumors with molecules carrying radioactive particles that bind to specific receptors expressed by the cancer. Lutetium Lu 177 dotatate may also be referred to as 177Lu-DOTA-Tyr3-octreotate.
Compared to the alternative somatostatin analog DOTA-Tyr3-octreotide (dotatoc), Lutetium Lu 177 dotatate displays a higher uptake of radioactivity in tumors and better residence times. In terms of biodistribution, Lutetium Lu 177 dotatate demonstrated lower whole-body retention, indicating a potentially lower risk for bone marrow toxicity. The presence of a radioligand allows monitoring of treatment response post-therapy and before the next fraction of the dose delivery, which may be clinically beneficial in estimating the intensity of lesion uptakes or deciding the dose for subsequent administrations.
- In January 2018, the FDA approved lutetium LUTATHERA, a radiolabeled somatostatin analog, for the treatment of SSTR+ GEP-Neuroendocrine Tumors, including foregut, midgut, and hindgut neuroendocrine tumors in adults. Approval was based on data from NETTER-1 (NCT01578239), a randomized, multicenter, open-label, active-controlled trial in 229 patients with progressive, well-differentiated, locally advanced/inoperable or metastatic somatostatin receptor-positive midgut carcinoid tumors.
- In April 2024, LUTATHERA was approved for the treatment of pediatric patients 12 years and older with SSTR+ GEP-Neuroendocrine Tumors, including foregut, midgut, and hindgut Neuroendocrine Tumors.
- SOMATULINE DEPOT (lanreotide): Ipsen Biopharmaceuticals
SOMATULINE DEPOT is a somatostatin analog indicated for the treatment of adult patients with unresectable well or moderately differentiated, locally advanced, or metastatic GEP-Neuroendocrine Tumors to improve progression-free survival. The drug is also approved for the treatment of adults with carcinoid syndrome by reducing the frequency of short-acting somatostatin analog rescue therapy. The drug is administered subcutaneously.
- In December 2014, Ipsen Biopharmaceuticals announced that SOMATULINE DEPOT was approved by the US FDA for the treatment of GEP-Neuroendocrine Tumors in adult patients with unresectable, well or moderately differentiated, locally advanced or metastatic disease to improve progression-free survival (PFS).
- In September 2017, the FDA approved a supplemental indication for SOMATULINE DEPOT injection 120 mg for the treatment of carcinoid syndrome and in June 2019 the FDA approved a new pre-filled syringe for SOMATULINE DEPOT.
- In May 2024, Cipla announced that it had received the final approval for its ANDA for lanreotide injection 120 mg/0.5 mL, 90 mg/0.3 mL, and 60 mg/0.2 mL from the US FDA.
Neuroendocrine Tumors Emerging Drugs
- ITM-11 (n.c.a. 177Lu-edotreotide): ITM Solucin GmbH
ITM-11 (177Lu-edotreotide) is known as an innovative targeted radionuclide therapy agent and is under investigation in the Phase III clinical trial COMPETE. ITM-11 shows a favorable safety profile and promising efficacy for the treatment of GEP-Neuroendocrine Tumors, for which only a few suitable and well-tolerated therapies are marketed. ITM-11 consists of two molecular components – firstly, Edotreotide (DOTATOC), an octreotide-derived somatostatin analog, and secondly, EndolucinBeta (no-carrier-added (n.c.a.) lutetium-177 chloride) a synthetic, low-energy beta-emitting therapeutic radioisotope.
ITM-11 received an orphan designation as a treatment for GEP-Neuroendocrine Tumors based on the data from a Phase II clinical study, which demonstrated a significant benefit (substantially improved progression-free survival, PFS). Furthermore, a uniquely low uptake by normal organs and a high tumor-to-kidney ratio were shown.
ITM-11 is currently being evaluated in ITM’s two Pivotal Phase III clinical trials, COMPETE and COMPOSE. While COMPETE (NCT03049189) is evaluating ITM-11 for the treatment of patients with Grade 1 and Grade 2 GEP-Neuroendocrine Tumors, the radiopharmaceutical candidate is also being investigated in COMPOSE (NCT04919226), for patients with well-differentiated high Grade 2 and Grade 3 GEP-Neuroendocrine Tumors.
- CAM2029: Camurus
CAM2029 is a long-acting octreotide subcutaneous depot under development for the treatment of three rare diseases: acromegaly, GEP-NET, and polycystic liver disease (PLD). CAM2029 is also ready to use and stored at room temperature. The randomized, active-controlled Pivotal Phase III SORENTO study evaluating the efficacy and safety of octreotide subcutaneous depot (CAM2029) in patients with GEP-Neuroendocrine Tumors progressed. Safety data collected to date was reviewed by a Data Monitoring Committee with no safety issues identified and the study recommended to continue without modifications.
According to the Q1 2024 financial report, NDA/MAA submission based on the SORENTO trial for GEP-Neuroendocrine Tumors is estimated in the second half of 2025. According to the Q2 2024 interim report, topline results of SORENTO trial for CAM2029 are anticipated in the first half of 2025.
Neuroendocrine Tumors Drugs Market Insights
SSTR agonists
SSTR agonists are internalized following high-affinity binding to their receptors and have traditionally been employed for in vivo targeting of SSTR receptors. This process is regarded as a crucial step in the in vivo targeting of receptors with SSTR agonists. The evolving PET/CT technology and the optimization of radiopharmaceutical chelation for effective somatostatin analog development opened the door to [68Ga]Ga-DOTA0-Tyr3-octreotate ([68Ga]Ga-DOTATATE) PET/CT. The standardized uptake value (SUV) of 68Ga-DOTA-SSA PET/CT in Neuroendocrine Tumors patients is related to the expression of SSTR2 and can serve as a distinct predictor of overall survival.
Neuroendocrine Tumors Market Outlook
For many patients with Neuroendocrine Tumors, the administration of SSAs represents the current standard of care. In addition to an antisecretory effect, this therapy is also considered to have antiproliferative properties. In numerous studies, the time to tumor progression and overall survival were better than with placebo therapy. Newer therapeutic options include the administration of targeted therapies such as the mTOR antagonist everolimus. In contrast, cytotoxic chemotherapy is ineffective in GI-NET. Just recently, the so-called PRRT was established as a novel therapeutic option in Neuroendocrine Tumors patients’ refractory to other treatments. Despite this progress, the prognosis of metastatic disease remains poor.
The NCCN and ENeuroendocrine Tumors guidelines recommend using an SSA as the first-line therapy when SSTR-related functional imaging is positive, the tumor has a slow progression rate, and the tumor grade is low. In cases where the tumor grade is higher, progression is more rapid, or SSTR imaging is negative or uneven, everolimus may be considered for first-line treatment. Otherwise, everolimus is typically used as a second-line therapy. For patients with lung Neuroendocrine Tumors that progress after treatment with an SSA and everolimus, options include chemotherapy (with various regimens), PRRT, locoregional treatments (primarily nonsurgical), or experimental therapies in clinical trials.
To summarize, as several new medicines enter the market, the treatment paradigm for Neuroendocrine Tumors is expected to evolve in the coming years. Demand for emerging therapies would be driven by physician enthusiasm, which a significant unmet need, frequent switching, and the usage of multi-drug regimens would fuel.
- The total Neuroendocrine Tumors Market Size in the US was estimated to be nearly USD 1,530 million in 2023, which is expected to increase due to the launch of emerging therapies.
- Among EU4 and the UK, the highest Neuroendocrine Tumors market share was found in the UK which was estimated to be nearly 31% of the market share in EU4 and the UK in 2023.
- In 2023, Somatostatin analogs (SSAs) captured the highest Neuroendocrine Tumors market size of approximately USD 700 million in the US, followed by LUTATHERA.
- To get significant market share, radiopharmaceutical key Neuroendocrine Tumors Companies need to ensure their product capabilities, supply, and production, and should be ready to meet the challenge of providing new therapeutic approaches to patients with cancer who otherwise have limited options.
- Oncolytic virotherapy holds promise for cancer treatment. Oncolytic virotherapies like ELC-100 (Elicera Therapeutics) and SVV-001 (Seneca therapeutics) are being investigated for the treatment of Neuroendocrine Tumors, however, it is too early predict their future owing to lack of efficacy and safety evidences in clinical trials.
Key Developments in the Neuroendocrine Tumors Treatment Market
- In April 2024, Perspective Therapeutics announced the selection of investigational product [212Pb]VMT-a-NET for the treatment of certain patients with neuroendocrine tumors by the US FDA to participate in the Chemistry, Manufacturing, and Controls Development and Readiness Pilot program.
- As per Crinetics Pharmaceuticals Q2 2024 quarter report published in August, the company anticipates the initiation of a Phase III program of paltusotine for carcinoid syndrome by the end of 2024, following consultation with the FDA.
- According to the Q2 2024 interim report of Camurus, topline results for CAM2029 are anticipated in the first half of 2025.
- BMS anticipates presenting registrational data for RYZ101 in second-line and beyond GEP-Neuroendocrine Tumors by 2026.
- In February 2024, BMS announced that it had completed its acquisition of RayzeBio. With the completion of the acquisition, RayzeBio shares have ceased trading on the NASDAQ Global Market and RayzeBio is now a wholly owned subsidiary of Bristol Myers Squibb. This transaction brings a promising pipeline of RPTs to Bristol Myers Squibb, including RayzeBio’s lead program RYZ101 (225Ac-DOTATATE), which targets SSTR2, over-expressed in GEP-Neuroendocrine Tumors and ES-SCLC.
Neuroendocrine Tumors Drugs Uptake
This section focuses on the uptake rate of potential Neuroendocrine Tumors drugs expected to be launched in the market during 2020–2034. The landscape of Neuroendocrine Tumors treatment market has experienced a profound transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing cancer care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Neuroendocrine Tumors Pipeline Development Activities
The Neuroendocrine Tumors therapeutics market report provides insights into therapeutic candidates in Phase III, Phase II, and Phase I/II. It also analyzes key Neuroendocrine Tumors Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Neuroendocrine Tumors therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Neuroendocrine Tumors emerging therapy.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on NET's evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including oncologists, radiation oncologists, surgical oncologists, and others.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the US and EU4 and the UK. Centers such as - Fukuoka Sanno Hospital, Smilow Cancer Hospital Yale Cancer Center, National Institutes of Health in the USA, Moffitt Cancer Center, MD Anderson Cancer Center Madrid, Moffitt Cancer Center in Florida, University of Miami, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Neuroendocrine Tumors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Neuroendocrine Tumors market and the unmet needs.
Neuroendocrine Tumors Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Neuroendocrine Tumors Therapeutics Market Access and Reimbursement
Globally, Neuroendocrine Tumors patient families spend an essential proportion of their household income on Neuroendocrine Tumors care. Neuroendocrine Tumors patients have to face a huge economic burden alone without any healthcare coverage or proper reimbursement policies.
LUTATHERA
Novartis Patient Support
- Novartis Patient Support is a comprehensive program that helps patients start and stay on treatment. It helps patients with:
- Navigating the insurance process: A dedicated Novartis Patient Support team will work with the provider to help navigate insurance coverage for the patient’s medication.
- Getting financial support: If the patient has private insurance, they could be eligible for Co-Pay Plus and pay as little as USD 25 for their LUTATHERA treatment. Limitations apply - Up to USD 15,000 throughout the treatment. Offer not valid under Medicare, Medicaid, or any other federal or state programs. Novartis reserves the right to rescind, revoke, or amend this program without notice.
- Answering questions across the treatment journey: Speak to a live Novartis Patient Support agent about what to expect before, during, or after treatment.
WELIREG
Savings Offer
- Eligible, privately insured patients may save on their out-of-pocket costs for their prescription for WELIREG. Not valid for patients who are uninsured or patients with Medicare or other Government Program insurance.
- Eligible, privately insured patients may pay as little as USD 5 per prescription on each qualifying prescription. Maximum program savings is USD 9,450 per patient. Coupons may be redeemed once every 21 days before the expiration date printed on the coupon, on each qualifying prescription up to a 90-day supply.
SOMATULINE DEPOT Co-pay Assistance Program
Eligible patients may pay as little as USD 0 per prescription.
Key Eligibility Criteria:
- The patient currently has commercial (private) health insurance that covers SOMATULINE DEPOT
- The patient also has no primary or secondary insurance coverage under any state or federal healthcare program
- The patient has a residency in the US
- The patient has a valid prescription for SOMATULINE DEPOT
Further detailed analysis of emerging therapies drug uptake in the report…
Neuroendocrine Tumors Therapeutics Market Report Scope
- The Neuroendocrine Tumors therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the Neuroendocrine Tumors epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Neuroendocrine Tumors market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the US and the EU4 and the UK drug outreach.
- The Neuroendocrine Tumors therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the US and the EU4 and the UK Neuroendocrine Tumors drugs market.
Neuroendocrine Tumors Therapeutics Market Report Insights
- Patient-based Neuroendocrine Tumors Market Forecasting
- Therapeutic Approaches
- Neuroendocrine Tumors Pipeline Drugs Market
- Neuroendocrine Tumors Market Size and Trends
- Existing and Future Neuroendocrine Tumors Drugs Market Opportunity
Neuroendocrine Tumors Therapeutics Market Report Key Strengths
- 11 Years Neuroendocrine Tumors Market Forecast
- Neuroendocrine Tumors Epidemiology Segmentation
- Key Cross Competition
- Neuroendocrine Tumors Drugs Uptake
- Key Neuroendocrine Tumors Market Forecast Assumptions
Neuroendocrine Tumors Therapeutics Market Report Assessment
- Current Neuroendocrine Tumors Treatment Market Practices
- Neuroendocrine Tumors Unmet Needs
- Neuroendocrine Tumors Pipeline Drugs Market Profiles
- Neuroendocrine Tumors Drugs Market Attractiveness
- Qualitative Analysis (SWOT Analysis and Conjoint Analysis)
FAQs
- What was the Neuroendocrine Tumors market size, the Neuroendocrine Tumors treatment market size by therapies, Neuroendocrine Tumors drugs market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of Neuroendocrine Tumors?
- What are the disease risks, burdens, and unmet needs of Neuroendocrine Tumors? What will be the growth opportunities across the countries concerning the patient population with Neuroendocrine Tumors?
- Who is the major competitor of LUTATHERA in the market?
- What are the current options for the treatment of Neuroendocrine Tumors? What are the current guidelines for treating Neuroendocrine Tumors in the US and Europe?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy
- The Neuroendocrine Tumors therapeutics market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Neuroendocrine Tumors drugs market.
- Insights on patient burden/disease Neuroendocrine Tumors prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Neuroendocrine Tumors drugs market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US and EU4 (Germany, France, Italy, and Spain) and the United Kingdom.
- Identifying strong upcoming players in the Neuroendocrine Tumors drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Neuroendocrine Tumors drugs market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Artilces:-
- Understanding Neuroendocrine Tumors (NETs): An In-Depth Look
- Neuroendocrine Tumors (NETs): Unraveling the Mystery of a Complex Cancer
- Unveiling the Future: Global Neuroendocrine Tumor Market Trends & Innovations
- Gastroenteropancreatic Neuroendocrine Tumours Market To Gain Substantial Momentum With Entrance of Novel Therapies
- Neuroendocrine Tumors Newsletter
- Latest DelveInsight Blogs

-01.png)
-01.png)