Oncolytic Virus Market Insights
Key Highlights
- Oncolytic viruses, which are known for infecting cancer cells, flourish inside these cells where they replicate quickly, causing cell destruction by bursting them open and exposing their contents to the immune system.
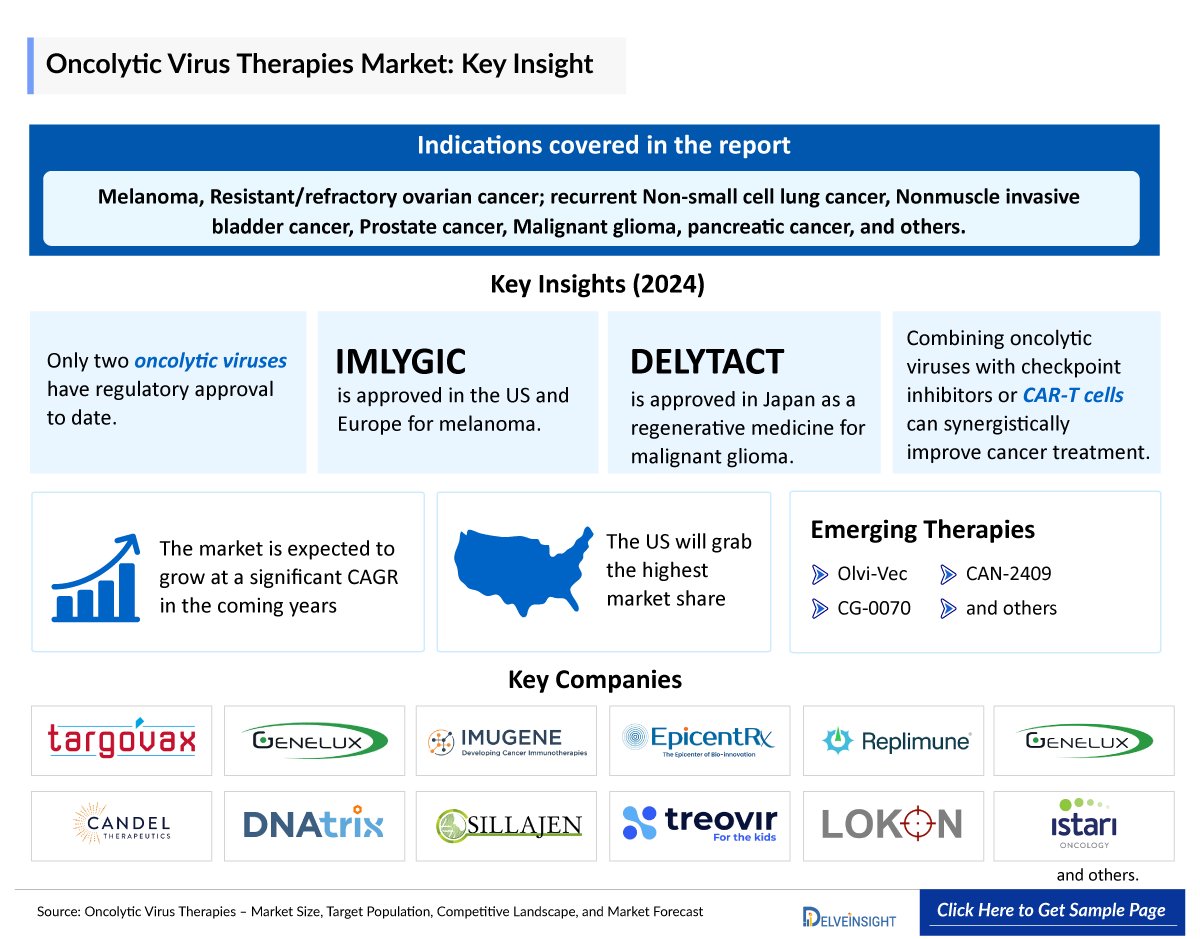
- Clinical trials have shown that OVs can kill cancer cells resistant to conventional therapies, potentially overcoming these challenges. To date, only two oncolytic viruses have received regulatory approval. talimogene laherparepvec (T-VEC), marketed as IMLYGIC, is approved in the US and Europe for the treatment of melanoma. In Japan, DELYTACT has been approved as a regenerative medicine for malignant glioma.
- A diverse array of OVs are currently in clinical development, with ongoing research focusing on factors such as route of administration and optimal combinations. Most trials are still in early phases, but the flexibility and specificity of OVs hold promise for regulatory approval.
- Combining oncolytic viruses with anti‐checkpoint antibodies such as anti‐PD‐1, anti‐PDL‐1, and anti‐CTLA4 or CAR‐T cells can be effective in cancer therapy in a synergistic fashion.
- Positive clinical trial results in treating melanomas, glioblastoma, ovarian cancer, pancreatic cancers, and many other solid tumors have significantly advanced the development of targeted therapies, including emerging oncolytic viruses.
- Active FDA involvement in oncolytic therapy and companies collabration signifies an upcoming advancement in the oncolytic virus therapy landscape and a huge change in cancer treatment with less side effects.
- For an instance in first half of the 2024 the FDA has issued Fast Track and Orphan Drug Designation to almost three oncolytic virus therapies.
- The pipeline for oncolytic virus therapies is rapidly expanding, with Genelux, Candel Therapeutics, CG Oncology, Lokon Pharma, Bioinvent, Istari Oncology, and many others leading the charge.
DelveInsight’s “Oncolytic Virus Therapies – Market Size, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the Oncolytic Virus Therapies, historical and Competitive Landscape as well as the oncolytic virus therapies market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Oncolytic Virus Therapies market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM oncolytic virus therapies market size from 2020 to 2034. The report also covers current oncolytic virus therapies treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Oncolytic Virus Therapies Understanding and Treatment Algorithm
Oncolytic Virus Therapies Overview
Oncolytic viral therapy is a treatment using a virus that can replicate to kill cancer cells. Many virus species exist, but not all can be designed to function as oncolytic viruses. Key characteristics of oncolytic viruses include non-pathogenicity, the ability to target and kill cancer cells specifically, and the potential to be genetically modified to express tumor-killing factors. Tumor selectivity could be concerned with the level of receptor-mediated cell entry, intracellular antiviral responses, or restriction factors that determine the susceptibility of the infected cell that leads to viral gene expression and replication.
The OV field gained considerable attention after positive results from many clinical trials. So far, four oncolytic viruses have been approved globally. The first OV, a picornavirus called RIGVIR, was approved in Latvia to treat melanoma but never achieved widespread use. Secondly, an engineered adenovirus, designated H101, was approved in China in 2005 to treat head and neck cancer. Thirdly, in 2015, another oncolytic viruses, an engineered Herpes simplex virus (HSV-1), named IMLYGIC (talimogene laherparepvec, (T-VEC)), was approved in the US and Europe for the treatment of non-resectable metastatic melanoma. Finally, in 2021, a modified herpes simplex virus, named DELYTACT, was approved in Japan for brain cancers such as glioblastoma.
Oncolytic Virus Therapies Treatment
n the coming decade, the oncolytic virus therapies landscape represents a huge potential for the pharmaceutical sector. Oncolytic virotherapy treatments, particularly in areas such as brain cancer, skin cancer, prostate cancer, and other kinds, have already begun to achieve therapeutic millstones as a result of recent advancements.
Oncolytic viruses represent a promising frontier in oncolytic virus therapies cancer therapy, harnessing the power of natural agents to seek out and eradicate malignant cells. These viruses possess an inherent preference for infiltrating, replicating within, and ultimately destroying cancer cells while sparing normal tissue.
Whether occurring naturally or engineered for enhanced efficacy, oncolytic viruses exhibit remarkable tumor selectivity, exploiting the unique vulnerabilities of cancer cells to trigger their demise. The therapeutic strategy of oncolytic virus therapy hinges on a dual mechanism: first, the targeted infection of tumor cells, followed by the induction of potent antitumor responses through the release of tumor antigens and stimulation of the host immune system. This process entails oncolytic viruses replicating within tumor cells, culminating in their lysis and the subsequent release of progeny virions, which can further propagate the infection to adjacent cancer cells, amplifying the cycle of cancer cell death.
Ultimately, oncolytic virus therapies hold great promise as a multifaceted approach that not only directly targets tumors but also mobilizes the immune system against cancer, offering a potent arsenal in the fight against malignancy.
Oncolytic Virus Therapies Drug Chapters
The drug chapter segment of the oncolytic virus therapies reports encloses a detailed analysis oncolytic virus therapies marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the oncolytic virus therapies' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug the latest news and press releases.
Oncolytic Virus Therapies Marketed Drugs
IMLYGIC (talimogene laherparepvec): Amgen
IMLYGIC is a genetically modified HSV-1 designed to replicate within tumors and produce an immunostimulatory protein called granulocyte-macrophage colony-stimulating factor (GM-CSF). IMLYGIC causes cell lysis, or death, which ruptures tumors, releasing tumor-derived antigens, which, along with GM-CSF, may promote an antitumor immune response; however, the exact mechanism of action is unknown. In October 2015, the FDA approved IMLYGIC for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery, an In December 2015, the European Commission approved for the treatment of adults with unresectable melanoma that is regionally or distantly metastatic (Stage IIIB, IIIC, and IVM1a), with no bone, brain, lung or other visceral disease.
DELYTACT (teserpaturev/G47Δ): Daiichi Sankyo
DELYTACT (teserpaturev/G47∆) is a genetically engineered oncolytic herpes simplex virus type 1 (HSV-1). DELYTACT has triple mutation within the viral genome that causes augmented and selective replication in cancer cells and enhanced induction of antitumor immune response while retaining high safety features. DELYTACT is currently the first third-generation oncolytic HSV-1 to be evaluated in humans. In June 2021, Daiichi Sankyo received conditional and time-limited approval from the Japan Ministry of Health, Labour and Welfare (MHLW) for DELYTACT (teserpaturev/G47∆), an oncolytic virus, for the treatment of patients with malignant glioma. It has received
|
Product |
Company |
Indication |
|
IMLYGIC (talimogene laherparepvec) |
Amgen |
Melanoma |
|
DELYTACT (teserpaturev/G47Δ) |
Daiichi Sankyo |
Malignant glioma |
Note: Detailed current therapies assessment will be provided in the full report of oncolytic virus therapies
Oncolytic Virus Therapies Emerging Drugs
Olvi-Vec (Olvimulogene nanivacirepvec): Genelux
Olvi-Vec is a modified vaccinia virus that utilizes a triple mode of action: it directly kills cancer cells, stimulates a tumor-specific immune response, and converts the tumor microenvironment from an immunosuppressive (cold state) to an immunoreactive (hot state). Currently, it is in clinical development for the treatment of ovarian cancer (resistant/refractory) and recurrent non-small cell lung cancer (NSCLC) in the US and China. Also, it is under clinical development for the same indication along with SCLC (recurrent). The US FDA has granted Fast Track Designation for the development program of olvi-vec for the treatment of patients with platinum-resistant/refractory ovarian cancer.
CG-0070 (Cretostimogene grenadenorepvec): CG Oncology
Cretostimogene is an investigational, intravesically delivered oncolytic immunotherapy being evaluated in BOND-003, a Phase III clinical trial for the treatment of Bacillus Calmette–Guerin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC). Cretostimogene is also being evaluated in a Phase III monotherapy clinical trial (PIVOT-006) in intermediate-risk NMIBC patients. In addition, cretostimogene is being evaluated in an investigator-sponsored clinical trial in combination with nivolumab for the treatment of muscle-invasive bladder cancer.
Note: Detailed emerging therapies assessment will be provided in the final report.
|
List of Emerging Drugs | |||||
|
Drug name |
Company |
Indication |
Virus type |
Phase |
Trial ID |
|
Olvi-Vec |
Genelux | Resistant/refractory ovarian cancer; recurrent Non-small cell lung cancer |
Vaccinia virus |
III |
NCT05281471 (OnPrime, GOG-3076) |
|
CG-0070 |
CG Oncology |
Nonmuscle invasive bladder cancer |
Adenovirus |
III |
NCT04452591 (BOND-003) |
|
CAN-2409 |
Candel Therapeutics |
Prostate, pancreatic, and lung cancer |
Herpes simplex virus thymidine kinase (HSV-tk) |
III |
NCT01436968 (PrTK03) |
Note: The emerging drug list is indicative, the full list will be given in the final report.
Oncolytic Virus Therapies Market Outlook
To date, only four oncolytic viruses have been approved for use as cancer treatments, though many more are currently in development. Several oncolytic virus have been discontinued either due to insufficient efficacy or due to unacceptable toxicity. Developing oncolytic virus presents numerous challenges, which may contribute to the difficulty in achieving regulatory approval.
In 2015, T-VEC (IMLYGIC), a genetically engineered HSV-1, became the first oncolytic virus approved in the USA for advanced unresectable melanoma and was subsequently approved in Europe. The most recent oncolytic virus, a modified HSV-1 called teserpaturev or G47Δ (DELYTACT), received conditional and time-limited approval in Japan in June 2021 for malignant gliomas. However, DELYTACT has not been approved in the United States.
The emerging therapies include olvimulogene nanivacirepvec, CAN-2409, cretostimogene grenadenorepvec, RP1, VG161, AdAPT-001, Voyager-V1, pelareorep, VCN-01, Pexa-Vec, delolimogene mupadenorepvec, BT-001, and T3011, which are currently in Phase III, Phase II, and Phase I/II trials.
The oncolytic virus therapy market is set for significant growth, providing new hope and innovative methods to combat cancer. The rapid pace of ongoing research, combined with collaborative efforts among scientists, clinicians, and industry stakeholders, will play a crucial role in shaping the future of this promising field in the forecast period (2024–2034). Advances in viral engineering, genetic modification, and personalized medicine are driving the development of more effective and targeted therapies.
Oncolytic Virus Therapies Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging oncolytic virus therapies expected to be launched in the market during 2020–2034.
Oncolytic Virus Therapies Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, and Phase II. It also analyzes key players involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for oncolytic virus therapies market growth over the forecasted period.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for oncolytic virus therapies emerging therapies.
The increasing strategic collaborations among major market players to enhance the growth of their pipeline products are anticipated to drive market expansion.
KOL Views
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on oncolytic virus therapies' evolving treatment landscape, patient reliance on conventional therapies, patient therapies switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM.
Their opinion helps understand and validate current and emerging therapies treatment patterns or oncolytic virus therapies market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Market Access and Reimbursement
IMLYGIC
IMLYGIC (talimogene laherparepvec) is a prescription medication used to treat a type of skin cancer called melanoma when it is on the skin or in lymph glands. IMLYGIC is a weakened form of herpes simplex virus type 1, which is commonly called the cold sore virus. The doctor will inject IMLYGIC directly into the tumor.
Amgen SupportPlus Copay Program
The Amgen SupportPlus Copay Program helps eligible commercially insured patients pay for their out-of-pocket prescription costs.
- Pay as little as USD 0 out-of-pocket for each dose or cycle
- Can be applied to deductible, co-insurance, and co-payment
- No income eligibility requirement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Key Updates on Oncolytic Virus Therapies
- In May 2024, Theriva Biologics announced that the US FDA has granted Fast Track Designation to VCN-01 in combination with gemcitabine and nab-paclitaxel to improve progression-free survival and overall survival in patients with metastatic pancreatic adenocarcinoma.
- In April 2024, ImmVira’s oncolytic product MVR-T3011 IT intratumoral injection received FDA Fast Track Designation for HNSCC treatment.
- In April 2024, Candel Therapeutics announced that the US FDA has granted Orphan Drug Designation to CAN-2409 for the treatment of pancreatic cancer
- In May 2024, Oncolytics Biotech entered a preliminary collaboration with the Global Coalition for Adaptive Research (GCAR). The purpose of the preliminary collaboration is to commence planning activities for the evaluation of pelareorep in the treatment of first-line metastatic pancreatic ductal adenocarcinoma (PDAC) as part of GCAR’s anticipated master protocol for metastatic pancreatic cancer
The abstract list is not exhaustive, will be provided in the final report
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of oncolytic virus therapies, explaining its mechanism, and therapies (current and emerging).
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the oncolytic virus therapies market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis expert insights/KOL views, and treatment preferences that help shape and drive the 7MM oncolytic virus therapies market.
Oncolytic Virus Therapies Report Insights
- Oncolytic Virus Therapies Targeted Patient Pool
- Oncolytic Virus Therapies Therapeutic Approaches
- Oncolytic Virus Therapies Pipeline Analysis
- Oncolytic Virus Therapies Market Size and Trends
- Existing and Future Market Opportunity
Oncolytic Virus Therapies Report Key Strengths
- Eleven years Forecast
- The 7MM Coverage
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
Oncolytic Virus Therapies Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions
- What was the oncolytic virus therapies total market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative market for oncolytic virus therapies?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for oncolytic virus therapies evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with oncolytic virus therapies? What will be the growth opportunities across the 7MM for the patient population on oncolytic virus therapies?
- What are the key factors hampering the growth of the oncolytic virus therapies market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for oncolytic virus therapies?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help develop business strategies by understanding the latest trends and changing dynamics driving the oncolytic virus therapies Market.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.





.jpg&w=3840&q=75)
