Overt Hepatic Encephalopathy Market Summary
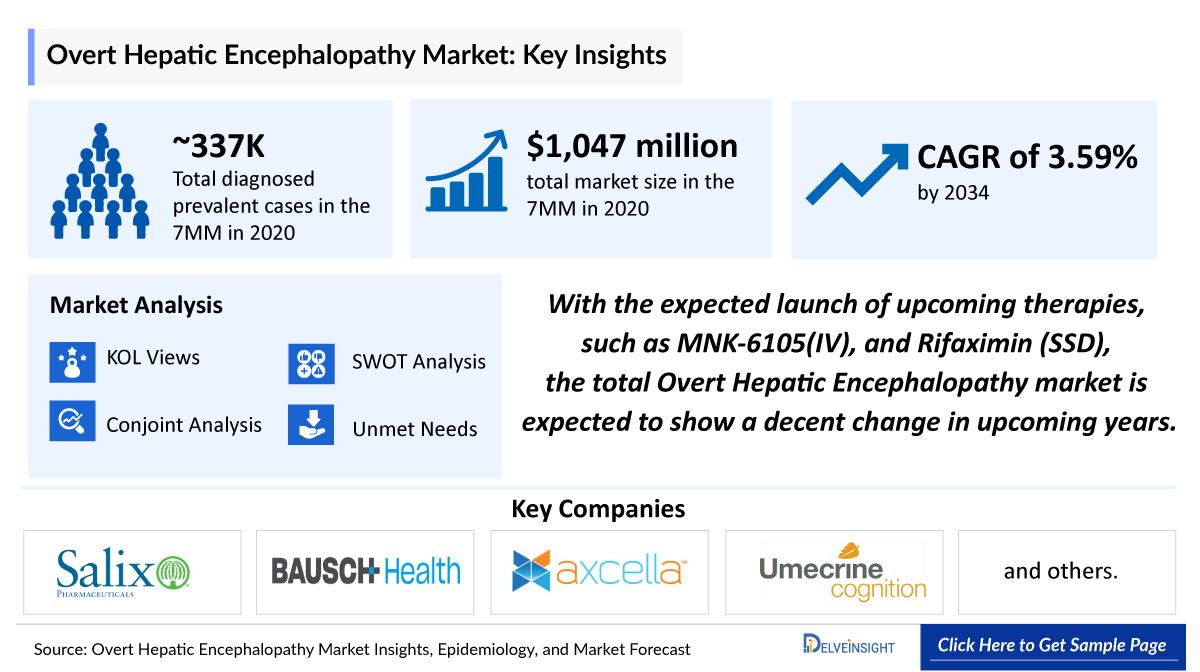
- The Overt Hepatic Encephalopathy Market Size was valued ~USD 1,047 million in 2020 and is anticipated to grow with a significnat CAGR of 3.59% during the study period (2020-2034).
- The leading Overt Hepatic Encephalopathy companies developig therapies include - Salix Pharmaceuticals, Bausch Health, Axcella Health, Inc., Umecrine Cognition, Mallinckrodt Therapeutics, Vedanta Biosciences, and Patricia Bloom, and others.
Overt Hepatic Encephalopathy Market and Epidemiology Analysis
- The Overt Hepatic Encephalopathy therapeutic market in the 7MM was USD 1,047 million in 2020.
- The Overt Hepatic Encephalopathy therapeutic market in the United States was USD 831 million in the year 2020.
- The market size in the 7MM might increase at a CAGR of 3.59%. An increase in the diagnosed prevalence of Overt Hepatic Encephalopathy and the expected approval of emerging therapies are the primary factors driving the growth of the Overt Hepatic Encephalopathy market.
- With the expected launch of upcoming therapies, such as MNK-6105(IV), and Rifaximin (SSD), the total Overt Hepatic Encephalopathy market is expected to show a decent change in upcoming years.
- The total diagnosed prevalent cases of Hepatic Encephalopathy in the 7MM were found to be 337,013 in 2020.
- The epidemiology model of Overt Hepatic Encephalopathy is based on the various published literature related to cirrhosis and its hospitalization. We also conducted interviews with the key opinion leaders (KOLs) to fill the gaps and confirm the findings of the secondary search.
- Assessments as per DelveInsight analysis, the US accounted for the majority of Hepatic Encephalopathy cases in the 7MM with 205,018 diagnosed prevalent cases in 2020.
Request for unlocking the Sample Page of the "Overt Hepatic Encephalopathy Market Insights"
Key Factors Driving Overt Hepatic Encephalopathy Market:
- Rising prevalence of chronic liver diseases: Increasing cases of cirrhosis, hepatitis, and alcohol-related liver disease are driving demand for overt hepatic encephalopathy treatments.
- Growing geriatric population: Aging populations are more susceptible to liver dysfunction and neurological complications, contributing to higher disease burden.
- Improved disease awareness and diagnosis: Better clinical recognition and standardized diagnostic guidelines are leading to increased treatment rates.
- Advancements in therapeutic options: Development of novel agents, combination therapies, and improved formulations of existing drugs are enhancing treatment outcomes.
- Supportive reimbursement and guideline-driven care: Favorable reimbursement policies and strong clinical guidelines are encouraging adoption of approved therapies.
DelveInsight's "Overt Hepatic Encephalopathy Market Insights, Epidemiology, and Market Forecast-2034" report delivers an in-depth understanding of the Overt Hepatic Encephalopathy, historical and forecasted epidemiology as well as the Overt Hepatic Encephalopathy therapeutics market trends in the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom) and Japan.
The Overt Hepatic Encephalopathy market report provides current treatment practices, emerging drugs, Overt Hepatic Encephalopathy market share of the individual therapies, current and forecasted Overt Hepatic Encephalopathy market size from 2020 to 2034 segmented by seven major markets. The Report also covers current Overt Hepatic Encephalopathy treatment practices/algorithms, market drivers, market barriers, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the Overt Hepatic Encephalopathy market.
Scope of the Overt Hepatic Encephalopathy Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Overt Hepatic Encephalopathy Market |
|
|
Overt Hepatic Encephalopathys Market Size | |
|
Overt Hepatic Encephalopathy Companies |
Salix Pharmaceuticals, Bausch Health, Axcella Health, Inc., Umecrine Cognition, Mallinckrodt Therapeutics, Vedanta Biosciences, and Patricia Bloom, and others. |
|
Overt Hepatic Encephalopathy Epidemiology Segmentation |
|
Overt Hepatic Encephalopathy Treatment Market
Hepatic encephalopathy is a brain disorder that develops in some individuals with liver disease. HE presents as a spectrum of neuropsychiatric symptoms ranging from subtle fluctuating cognitive impairment to coma and is a significant contributor to morbidity in patients with liver disease. It is observed in acute liver failure, liver bypass procedures – for example, shunt surgery and trans-jugular intrahepatic portosystemic shunt – and cirrhosis, classified as Type A, B, and C HE, respectively. Pathogenesis is linked with ammonia and glutamine production.
Moreover, Hepatic encephalopathy can be associated with more severe symptoms, including reduced alertness, shortened attention span, disruptions in sleep patterns, mild confusion, slowing ability to perform mental tasks, and mood or personality changes. More noticeable changes in memory, concentration, or intellectual function than in minimal hepatic encephalopathy (MHE) may also be observed. HE can also be classified according to whether its presence is overt or covert. When affected individuals have obvious, outward signs and symptoms, the disorder may be referred to as Overt Hepatic Encephalopathy.
Overt Hepatic Encephalopathy Diagnosis
The HE diagnosis is based on the presence of a spectrum of neuropsychiatric abnormalities in patients with liver dysfunction after excluding unrelated neurologic and/or metabolic causes of encephalopathy. The diagnosis of Overt Hepatic Encephalopathy is made after excluding other causes of encephalopathy and mental status changes. In addition to making the diagnosis of Overt Hepatic Encephalopathy, it is imperative to identify and address precipitating factors of Overt Hepatic Encephalopathy, such as infection, upper gastrointestinal bleeding, medications, or electrolyte/volume disturbances.
Overt Hepatic Encephalopathy Treatment
Management of Overt Hepatic Encephalopathy includes treating patients with acute HE episodes, preventing HE recurrence, and identifying and managing precipitating factors associated with HE development. The two primary forms of medical therapy for HE are nonabsorbable disaccharides (i.e., lactitol and lactulose) and nonabsorbable antibiotics (i.e., rifaximin). The hyperammonemia and inflammation that occur due to urea breakdown in cirrhotics have led to the development of HE treatments that target gut bacteria. Treatments for Overt Hepatic Encephalopathy are approved by the US FDA and some unapproved treatments are available for use. It is important to remember that in patients with cirrhosis and portosystemic shunting, skeletal muscle mass, and renal clearance are vital to neurotoxin clearance.
Overt Hepatic Encephalopathy Epidemiology
The Overt Hepatic Encephalopathy epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Prevalent Cases of Hepatic Encephalopathy, Gender-specific Diagnosed Prevalent Cases of Hepatic Encephalopathy, Age-specific Diagnosed Prevalent Cases of Hepatic Encephalopathy, and Type-specific Diagnosed Prevalent Cases of Hepatic Encephalopathy in the 7MM covering the United States, EU5 countries (Germany, France, Italy, Spain, and the United Kingdom), and Japan from 2020-2034.
Overt Hepatic Encephalopathy Epidemiology Key Findings
- The total diagnosed prevalent cases of Hepatic Encephalopathy in the 7MM were found to be 337,013 in 2020.
- The epidemiology model of Overt Hepatic Encephalopathy is based on the various published literature related to cirrhosis and its hospitalization. We also conducted interviews with the key opinion leaders (KOLs) to fill the gaps and confirm the findings of the secondary search.
- Assessments as per DelveInsight analysis, the US accounted for the majority of Hepatic Encephalopathy cases in the 7MM with 205,018 diagnosed prevalent cases in 2020.
- The age‐specific data revealed that the highest number of Hepatic Encephalopathy people affected with was found in the age group of 45–54 years, while people <25 years are the least affected.
- Assessments as per DelveInsight’s analysts show that the majority of cases of Hepatic Encephalopathy are males. There was a total of 149,048 male and 55,970 female cases of Hepatic Encephalopathy in 2020 in the United States.
- HE is divided into two broad categories based on severity, Covert Hepatic Encephalopathy (CHE) and Overt Hepatic Encephalopathy. There was a total of 82,007 Overt Hepatic Encephalopathy cases in 2020 in the United States which is expected to increase in the upcoming years.
- In EU-5, the United Kingdom has the highest number of cases of Overt Hepatic Encephalopathy with 10,971 cases in 2020, followed by Germany with 10,669 cases and France with 8,910 cases. While Spain reported the least number of cases i.e. 3,923 in 2020.
- In 2020, Japan had 11,829 diagnosed prevalent cases of Overt Hepatic Encephalopathy.
Country-Wise Overt Hepatic Encephalopathy Epidemiology
The Country-Wise Overt Hepatic Encephalopathy Epidemiology reveals significant variations in Overt Hepatic Encephalopathy prevalence across regions. These disparities highlight the need for region-specific healthcare strategies. Understanding the Overt Hepatic Encephalopathy prevalence helps healthcare providers and policymakers allocate resources effectively and improve patient outcomes in diverse geographic populations.
Overt Hepatic Encephalopathy Epidemiology Segmentation
- Diagnosed Prevalent Cases of Overt Hepatic Encephalopathy in the 7MM
- Type-specific Diagnosed Prevalent Cases of Overt Hepatic Encephalopathy in the 7MM
- Gender-specific Diagnosed Prevalent Cases of Overt Hepatic Encephalopathy in the 7MM
- Age-specific Diagnosed Prevalent Cases of Overt Hepatic Encephalopathy in the 7MM
Overt Hepatic Encephalopathy Drug Analysis
The drug chapter segment of the Overt Hepatic Encephalopathy report encloses a detailed analysis of Overt Hepatic Encephalopathy marketed drugs, mid-phase, and late-stage Overt Hepatic Encephalopathy pipeline drugs. It also helps to understand the Overt Hepatic Encephalopathy clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details of each included drug and the latest news and press releases.
Overt Hepatic Encephalopathy Marketed Drugs
Xifaxan: Salix Pharmaceuticals/Bausch Health
Xifaxan (rifaximin) tablets (550 mg) developed and marketed by Salix Pharmaceuticals (a subsidiary of Valeant Pharmaceuticals), were indicated for the reduction in risk of Overt Hepatic Encephalopathy (HE) recurrence in patients ≥ 18 years of age. It is also being marketed to treat Travelers’ Diarrhea and IBS-D (Irritable Bowel Syndrome with Diarrhea). Salix is exploring the potential additional indications, formulations, and clinical trials, and co-promotion arrangements for Xifaxan to capitalize on the potential for Xifaxan, including development programs in Crohn’s disease and liver disease.
Products details in the report…
Overt Hepatic Encephalopathy Emerging Drugs
AXA1665: Axcella Health, Inc.
AXA1665—Axcella’s product candidate for a reduction in risk of recurrent Overt Hepatic Encephalopathy—is a composition of eight amino acids and derivatives designed to target multiple metabolic pathways intersecting key organ systems, including the liver, muscle, and gut. In prior clinical studies, this oral product candidate was safe, and well-tolerated, and demonstrated the potential to improve ammonia handling, physical function, amino acid balance, and neurocognition with a safe and well-tolerated profile. Axcella conducted two studies for AXA1665: AXA1665-001 and AXA1665-002.
Products detail in the report…
GR3027 (Golexanolone): Umecrine Cognition
Umecrine Cognition, a Karolinska Development (KDEV), is developing novel GR3027 (golexanolone), an orally administrated small molecule to treat patients diagnosed with HE. Golexanolone is a GABAA-receptor modulating steroid antagonist (GAMSA) designed to antagonize positive GABAA-receptor modulation by endogenous neuroactive steroids.
List to be continued in the report…
Overt Hepatic Encephalopathy Market Outlook
Overt Hepatic Encephalopathy may develop over hours or days and occur spontaneously or following an event, such as gastrointestinal bleeding, infection, dehydration, or constipation.
First-line therapy for patients experiencing an acute episode of Overt Hepatic Encephalopathy is the nonabsorbable disaccharide lactulose, which requires self-titration to two to three soft bowel movements per day.
The Second-line of therapy for the management of HE includes probiotics, polyethylene glycol 3350–electrolyte solution (PEG), ammonia scavengers, branched-chain amino acids (BCAA), protein restriction, zinc, and fecal microbiota transplant (FMT).
The role of nutrition in the management of HE has been the focus of renewed attention. An expert panel commissioned by the International Society for Hepatic Encephalopathy and Nitrogen Metabolism recommended that all patients with cirrhosis and HE should undergo baseline nutritional assessment and interval reassessments as part of management planning. Protein restriction should never be recommended in patients with cirrhosis (with or without HE).
The availability of well-established healthcare infrastructure in the United States also contributes to the growth of the market. In addition, an increasingly aging population and increased R&D investments by drug manufacturing companies are fueling the market expansion
According to DelveInsight, the Overt Hepatic Encephalopathy market size and share in the 7MM is expected to change in the study period 2020-2034.
Overt Hepatic Encephalopathy Market Key Findings
- The Overt Hepatic Encephalopathy therapeutic market in the 7MM was USD 1,047 million in 2020.
- The Overt Hepatic Encephalopathy therapeutic market in the United States was USD 831 million in the year 2020.
- The first-line agent used to prevent acute or persistent Overt Hepatic Encephalopathy is the non-absorbable disaccharides (lactulose or lactitol). Extensive clinical experience has demonstrated the efficacy of oral non-absorbable disaccharides. Unfortunately, not all patients can tolerate these agents. The market size of Lactulose was USD 216 million in 2020.
- The market size of Xifaxan (Rifaximin) approved for the reduction in risk of Overt Hepatic Encephalopathy recurrence in patients ≥ 18 years of age was USD 383 million in 2020.
- The market size in the 7MM might increase at a CAGR of 3.59%. An increase in the diagnosed prevalence of Overt Hepatic Encephalopathy and the expected approval of emerging therapies are the primary factors driving the growth of the Overt Hepatic Encephalopathy market.
- With the expected launch of upcoming therapies, such as MNK-6105(IV), and Rifaximin (SSD), the total Overt Hepatic Encephalopathy market is expected to show a decent change in upcoming years.
- The Overt Hepatic Encephalopathy therapeutic market in Japan was USD 21 million in the year 2020.
The United States Overt Hepatic Encephalopathy Market Outlook
This section provides the total Overt Hepatic Encephalopathy market size and market size by therapies in the United States.
EU-5 Overt Hepatic Encephalopathy Market Outlook
The total Overt Hepatic Encephalopathy market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom are provided in this section.
Japan Overt Hepatic Encephalopathy Market Outlook
The total Overt Hepatic Encephalopathy market size and market size by therapies in Japan are provided.
Overt Hepatic Encephalopathy Competitive Landscape
The Overt Hepatic Encephalopathy competitive landscape is moderately concentrated yet evolving, anchored by well‑established treatments and increasing innovation from specialty firms. Traditional therapies like lactulose (first‑line non‑absorbable disaccharide) and rifaximin (broad‑spectrum, non‑absorbed antibiotic) remain dominant in clinical practice, with Bausch Health Companies Inc. (Salix) leading the market through widespread rifaximin adoption and entrenched guideline use. AbbVie, Fresenius Kabi, and regional generics manufacturers such as Lupin and Dr. Reddy’s compete strongly in lactulose and cost‑effective alternatives, especially in hospital and outpatient settings.
Beyond these core players, emerging biopharmaceutical and specialty companies including Cosmo Pharmaceuticals, Umecrine Cognition, Rebiotix (Ferring) and others are pursuing novel mechanisms such as microbiome‑based therapies, GABAergic modulation, and advanced ammonia‑lowering approaches to differentiate future offerings. Competitive strategies include portfolio diversification, lifecycle management of existing products, strategic collaborations, and regional expansion into high‑growth markets. This combination of established dominance and early‑stage innovation shapes a dynamic, multi‑tiered competitive environment in the OHE market.
Key Overt Hepatic Encephalopathy Companies
- The Key Overt Hepatic Encephalopathy companies actively involved in the Overt Hepatic Encephalopathy treatment landscape include -
- Salix Pharmaceuticals
- Bausch Health
- Axcella Health, Inc.
- Umecrine Cognition
- Mallinckrodt Therapeutics
- Vedanta Biosciences
- Patricia Bloom, and others
Overt Hepatic Encephalopathy Drugs Uptake
This section focuses on the rate of uptake of the potential drugs recently launched in the Overt Hepatic Encephalopathy market or expected to get launched in the market during the study period 2020-2034. The analysis covers Overt Hepatic Encephalopathy market uptake by drugs, patient uptake by therapies, and sales of each drug.
This helps in understanding the drugs with the most rapid uptake, and reasons behind the maximal use of new drugs, and allows the comparison of the drugs based on market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Overt Hepatic Encephalopathy Development Activities
The Overt Hepatic Encephalopathy pipeline report provides insights into different Overt Hepatic Encephalopathy clinical trials within phase II, and phase III stages. It also analyzes key players involved in developing targeted therapeutics.
Overt Hepatic Encephalopathy Pipeline Activities
The Overt Hepatic Encephalopathy clinical trials analysis report covers detailed information of collaborations, acquisitions, mergers, licensing, and patent details for Overt Hepatic Encephalopathy emerging therapies.
Overt Hepatic Encephalopathy Market Reimbursement Scenario
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In the report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
KOL- Views on Evolving Overt Hepatic Encephalopathy Market
To keep up with current Overt Hepatic Encephalopathy market trends, we take KOLs and SMEs ' opinion working in the Overt Hepatic Encephalopathy domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Overt Hepatic Encephalopathy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Overt Hepatic Encephalopathy Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Overt Hepatic Encephalopathy by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Overt Hepatic Encephalopathy Market Report
- The report covers the descriptive overview of Overt Hepatic Encephalopathy, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
- Comprehensive insight has been provided into the Overt Hepatic Encephalopathy epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Overt Hepatic Encephalopathy is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Overt Hepatic Encephalopathy market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the global Overt Hepatic Encephalopathy market
Overt Hepatic Encephalopathy Market Report Highlights
- In the coming years, the Overt Hepatic Encephalopathy market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Overt Hepatic Encephalopathy R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for Overt Hepatic Encephalopathy. The launch of emerging therapies will significantly impact the Overt Hepatic Encephalopathy market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Overt Hepatic Encephalopathy
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Overt Hepatic Encephalopathy Market Report Insights
- Overt Hepatic Encephalopathy Patient Population
- Overt Hepatic Encephalopathy Therapeutic Approaches
- Overt Hepatic Encephalopathy Pipeline Analysis
- Overt Hepatic Encephalopathy Market Size and Trends
- Overt Hepatic Encephalopathy Market Opportunities
- Impact of Upcoming Overt Hepatic Encephalopathy Therapies
Overt Hepatic Encephalopathy Market Report Key Strengths
- 10 Years Forecast
- 7MM Coverage
- Overt Hepatic Encephalopathy Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Overt Hepatic Encephalopathy Market
- Overt Hepatic Encephalopathy Drugs Uptake
Overt Hepatic Encephalopathy Market Report Assessment
- Current Overt Hepatic Encephalopathy Treatment Practices
- Overt Hepatic Encephalopathy Unmet Needs
- Overt Hepatic Encephalopathy Pipeline Product Profiles
- Overt Hepatic Encephalopathy Market Attractiveness
- Overt Hepatic Encephalopathy Market Drivers
- Overt Hepatic Encephalopathy Market Barriers
Key Questions Answered In The Overt Hepatic Encephalopathy Market Report:
Overt Hepatic Encephalopathy Market Insights:
- What was the Overt Hepatic Encephalopathy drug class share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Overt Hepatic Encephalopathy total market size as well as market size by therapies across the 7MM during the forecast period (2020-2034)?
- What are the key findings pertaining to the market across 7MM and which country will have the largest Overt Hepatic Encephalopathy market size during the forecast period (2020-2034)?
- At what CAGR, the Overt Hepatic Encephalopathy market is expected to grow by 7MM during the forecast period (2020-2034)?
- What would be the Overt Hepatic Encephalopathy market outlook across the 7MM during the forecast period (2020-2034)?
- What would be the Overt Hepatic Encephalopathy market growth till 2034, and what will be the resultant market Size in the year 2034?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Overt Hepatic Encephalopathy Epidemiology Insights:
- What are the disease risk, burden, and regional/ethnic differences of the Overt Hepatic Encephalopathy?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What is the historical Overt Hepatic Encephalopathy patient pool in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What would be the forecasted patient pool of Overt Hepatic Encephalopathy in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- Where will be the growth opportunities in the 7MM with respect to the patient population pertaining to Overt Hepatic Encephalopathy?
- Out of all 7MM countries, which country would have the highest prevalent population of Overt Hepatic Encephalopathy during the forecast period (2020-2034)?
- At what CAGR the patient population is expected to grow in 7MM during the forecast period (2020-2034)?
Current Overt Hepatic Encephalopathy Treatment Scenario, Marketed Drugs and Emerging Therapies:
- What are the current options for the Overt Hepatic Encephalopathy treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of Overt Hepatic Encephalopathy in the USA, Europe, and Japan?
- What are the Overt Hepatic Encephalopathy marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety and efficacy, etc.?
- How many companies are developing therapies for the treatment of Overt Hepatic Encephalopathy?
- How many therapies are in development by each company for Overt Hepatic Encephalopathy treatment?
- How many are emerging therapies in the mid-stage, and late stages of development for Overt Hepatic Encephalopathy treatment?
- What are the key collaborations (Industry - Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to Overt Hepatic Encephalopathy therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for Overt Hepatic Encephalopathy and their status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for Overt Hepatic Encephalopathy?
- What are the global historical and forecasted market of Overt Hepatic Encephalopathy?
Reasons to buy Overt Hepatic Encephalopathy Market Forecast Report
- The report will help in developing business strategies by understanding trends shaping and driving the Overt Hepatic Encephalopathy market
- To understand the future market competition in the Overt Hepatic Encephalopathy market and Insightful review of the key market drivers and barriers
- Organize sales and marketing efforts by identifying the best opportunities for Overt Hepatic Encephalopathy in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom) and Japan
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for Overt Hepatic Encephalopathy market
- To understand the future market competition in the Overt Hepatic Encephalopathy market