Parkinson's Disease Levodopa-Induced Dyskinesia Market
- As people age, the occurrence of Parkinson's disease tends to rise, particularly among the elderly demographic. Consequently, given the prolonged duration of the disease in affected individuals, the probability of developing Levodopa-induced Dyskinesia (LID) over time also amplifies. Therefore as per our estimation, the Parkinson’s disease Levodopa-induced Dyskinesia cases are expected to increase during the forecast period (2024-2034).
- Evolving Parkinson's Disease Levodopa-induced Dyskinesia Treatment guidelines may lead to more aggressive initiation of levodopa therapy in Parkinson’s disease patients, particularly in the early stages of the disease. While this can improve motor symptoms initially, it may also increase the risk of developing LID over time.
- Current Parkinson's Disease Levodopa-induced Dyskinesia Treatment typically involve a combination of pharmacological, surgical, and non-pharmacological approaches aimed at managing motor symptoms and improving the patient's quality of life.
- Supernus Pharmaceuticals' GOCOVRI received approval in 2021 for managing OFF episodes in Parkinson's disease patients treated with levodopa who experience dyskinesia and other motor complications. However, reported adverse effects include hallucinations, dizziness, dry mouth, peripheral edema, constipation, falls, and orthostatic hypotension.
- While there are several medications and therapeutic approaches available for managing PD-LID, none are entirely effective for all patients, and the options for managing dyskinesias are somewhat limited. This highlights the need for continued research and development of novel therapies targeting dyskinesias specifically.
- Furthermore, the response to levodopa and the development of dyskinesias vary widely among PD patients. Some individuals may experience dyskinesias early in the disease course, while others may develop them after years of levodopa therapy. This variability makes it challenging to predict and manage dyskinesias effectively.
- However, several Parkinson's Disease Levodopa-induced Dyskinesia Companies are working robustly on many new therapies, including NLX-112 (Neurolixis SAS/Gala laboratories), JM-010 (Bukwang Pharmaceutical/Contera Pharma), KETARX (ketamine) (PharmaTher), AV-101 (Vistagen), and others anticipated to positively influence the market size Parkinson’s disease Levodopa-induced Dyskinesia during the forecast period [2024-2034].
Request for Unlocking the Sample Page of the "Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market"
DelveInsight’s “Parkinson's Disease Levodopa-induced Dyskinesia Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Parkinson’s Disease Levodopa-induced Dyskinesia, historical and forecasted epidemiology, as well as the Parkinson’s disease Levodopa-induced Dyskinesia market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Parkinson’s disease Levodopa-induced Dyskinesia market size from 2020 to 2034. The report also covers Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2019 to 2032 |
|
Forecast Period |
2023-2032 |
|
Geographies Covered |
|
|
Parkinson's Disease Levodopa-induced Dyskinesia Market |
|
|
Parkinson's Disease Levodopa-induced Dyskinesia Market Size | |
|
Parkinson's Disease Levodopa-induced Dyskinesia Companies |
|
Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market: Understanding and Algorithm
Parkinson’s disease (PD) is a life-threatening progressive neurodegenerative disease characterized by severe locomotor impairments, including bradykinesia, tremor, and rigidity. Other abnormalities associated with PD are cognitive defects, psychiatric abnormalities, and neurodegenerative implications of levodopa-induced dyskinesia (LID). Levodopa (L-DOPA) is highly efficient and is used to mitigate PD, but its prolonged use gives rise to motor abnormalities, including dyskinesia. Dyskinesia may be mild at first but may develop into a debilitating symptom and affect the quality of life of patients. More than 50% of patients with PD develop LID after 5 years of continuous treatment with L-DOPA, which worsens the quality of life in these patients.
LID is clinically heterogeneous; they commonly present as chorea or choreoathetosis, though myoclonus, akathisia, ballism, and other abnormal movements have also been described. Uncommon forms of LID include akathisia (excessive motor restlessness), a high-stepped overshooting gait, rapid alternating movements (RAM) of legs, blepharospasm, and a mixed pattern of abnormal movements.
The pathophysiology of LIDs is likely to involve alterations in the level of postsynaptic dopamine receptors since such receptors are relatively preserved in PD as opposed to atypical parkinsonian syndromes where the degenerative neuronal process involves the postsynaptic striatal neurons. The development of dyskinesia has been associated with several risk factors, such as; the age of the onset of PD, PD severity, duration of the levodopa treatment, daily dosage of levodopa, and the use of levodopa vs. dopamine agonists.
Continued in the report...
Parkinson’s Disease Levodopa-induced Dyskinesia diagnosis
The diagnosis of Parkinson’s disease Levodopa-induced Dyskinesia typically involves a thorough clinical evaluation by a neurologist or movement disorder specialist. Various scales and instruments have been developed to objectively assess LID and its impact on patients' overall quality of life. These include the rush dyskinesia rating scale (RDRS), this scale evaluates the severity of dyskinesias across different body parts and activities. Similar to the RDRS is the unified dyskinesia rating scale, this scale assesses the severity and impact of dyskinesias on daily activities. Another tool used to evaluate the severity of dyskinesias in Parkinson's disease patients is the clinical dyskinesia rating scale (CDRS). Furthermore, to measure the impact of dyskinesia on quality of life, instruments such as the Parkinson's disease Questionnaire (PDQ-39) and the Parkinson's disease Quality of Life Scale are commonly employed. These tools help quantify the subjective experience of living with Parkinson's disease and its associated complications, including dyskinesias. Additionally, patients' self-evaluation diaries, like the Hauser diary, are utilized to understand the effects of medications used to treat LID. However, maintaining compliance with diary completion and ensuring accuracy can be challenging.
Furthermore, several quantitative instrumental techniques have been developed to objectively quantify dyskinesias. These include wearing devices such as accelerometers and position transducers, which can track movements and provide quantitative data on dyskinesia severity and frequency. These objective measurements complement subjective assessments and provide valuable insights into the effectiveness of treatments and their impact on patients' daily lives.
Continued in the report...
Parkinson’s Disease Levodopa-induced Dyskinesia treatment
The treatment of Parkinson’s disease Levodopa-induced Dyskinesia typically involves a combination of pharmacological, surgical, and non-pharmacological approaches aimed at managing motor symptoms and improving the patient's quality of life. Dopaminergic medications, COMT inhibitors, amantadines, and others are used to help reduce dyskinesias while maintaining motor control.
Furthermore, the treatment strategy includes identifying the kind of dyskinesia and tailoring treatment accordingly. Peak-dose dyskinesia is treated mainly by reducing individual doses of levodopa and adding amantadine and dopamine agonists, whereas off-period dystonia often responds to baclofen and botulinum toxin injections. Diphasic dyskinesias are the most difficult to treat, particularly in patients with young-onset PD. While fractionation of levodopa dosage is the most frequently utilized strategy, many patients require DBS to control their troublesome motor fluctuations and LIDs.
Parkinson’s disease Levodopa-induced Dyskinesia Epidemiology
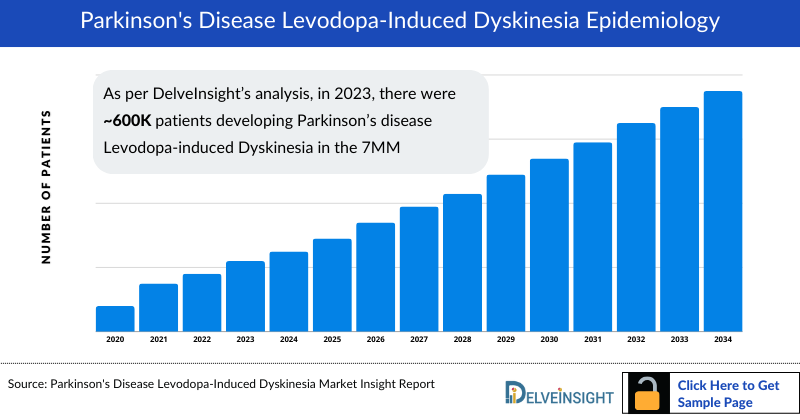
As the market is derived using a patient-based model, the Parkinson’s disease Levodopa-induced Dyskinesia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by number of patients developing Parkinson’s Disease Levodopa-induced Dyskinesia, and type-specific cases of Parkinson’s Disease Levodopa-induced Dyskinesia in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- As per DelveInsight’s analysis, in 2023, there were approximately 600 thousand patients developing Parkinson’s disease Levodopa-induced Dyskinesia in the 7MM, out of which the US accounted for nearly 300 thousand cases. These cases are expected to increase during the forecast period (2024-2034) driven by the advancements in medical understanding, diagnostic methodologies, and heightened awareness surrounding Parkinson's disease.
- In 2023, among the EU4 and the UK, Germany accounted for the highest number of patients developing Parkinson’s disease Levodopa-induced Dyskinesia i.e. approximately 67 thousand followed by France, and Italy with nearly 56 thousand, and 47 thousand respectively.
- In 2023, around 240 thousand Parkinson’s disease Levodopa-induced Dyskinesia-affected individuals were associated with peak-dose dyskinesia, 88 thousand with off-period dystonia, and 59 thousand with diphasic dyskinesia in the US. These cases are expected to change by 2034 owing to the aging population that is likely to experience Parkinson’s disease Levodopa-induced Dyskinesia.
- In 2023, Japan accounted for approximately 6% of cases of patients developing Parkinson’s disease Levodopa-induced Dyskinesia. Out of which nearly 35 thousand, 13 thousand, and 8 thousand Parkinson’s disease Levodopa-induced Dyskinesia-affected individuals were associated with peak-dose dyskinesia, off-period dystonia, and diphasic dyskinesia. It is estimated that these cases are expected to increase during the forecast period with the prevalence of the aging population in Japan, the number of individuals diagnosed with Parkinson's disease is expected to rise, and with longer disease duration, more patients are likely to experience complications such as LID.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Parkinson's Disease Levodopa-induced Dyskinesia Prevalence
Parkinson’s disease Levodopa-induced Dyskinesia Drug Chapters
The drug chapter segment of the Parkinson’s disease Levodopa-induced Dyskinesia Drugs Market Report encloses a detailed analysis of Parkinson’s disease Levodopa-induced Dyskinesia marketed drugs and late-stage (Phase III and Phase II) Parkinson's Disease Levodopa-induced Dyskinesia pipeline drugs analysis. It also helps understand the Parkinson’s disease Levodopa-induced Dyskinesia clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Parkinson's Disease Levodopa-induced Dyskinesia Marketed Drugs
- GOCOVRI: Supernus Pharmaceuticals
GOCOVRI contains amantadine in an extended-release formulation for oral use; the active ingredient in GOCOVRI is amantadine hydrochloride, the chemical name of which is tricycle decan-1-amine, hydrochloride, or 1-adamantanamine hydrochloride. The mechanism by which amantadine exerts efficacy in treating dyskinesia in patients with PD or as adjunctive treatment to levodopa/carbidopa in patients with PD experiencing “off” episodes is unknown. It might work by reducing excessive glutamatergic activity, which contributes to dyskinesia and OFF. Amantadine may have direct and indirect effects on dopamine neurons; it exerts dopaminergic-like side effects, such as hallucinations and dizziness in humans.
Note: Further marketed drugs and their details will be provided in the report...
Parkinson's Disease Levodopa-induced Dyskinesia Emerging Drugs
- JM-010: Bukwang Pharmaceutical/Contera Pharma
JM-010 is a combination of buspirone, a 5-HT1A agonist, and zolmitriptan, a 5-HT1B/5-HT1D agonist. JM-010 acts as a dual molecular switch by which the target efficacies of two existing and safe medications are perfectly fine-tuned to treat disease. JM-010 targets central mechanisms underlying the development of dyskinesia in PD. JM-010 has successfully demonstrated its efficacy and safety in preclinical studies, as well as in Phase I clinical safety and Phase II clinical proof of concept studies. JM-010 is being evaluated in Phase II clinical studies in Europe and the US.
- NLX-112 (befiradol): Neurolixis SAS/Gala laboratories
NLX-112 (also known as befiradol or F13640) is a novel compound that activates serotonin 5-HT1A receptors. Inhibition of serotonergic neurons is a promising strategy to diminish dyskinesia in PD and can be achieved by targeting serotonin 5-HT1A receptors, which inhibit serotonergic function. NLX-112 is highly effective in reducing abnormal movements in animal models of PD, and based on these data, Neurolixis is conducting clinical trials in PD patients who exhibit LID. The company completed a Phase II clinical trial for Parkinson’s disease Levodopa-induced Dyskinesia, which indicated NLX-112 was well tolerated in patients and significantly reduced their dyskinesias. Neurolixis is now looking to advance the development of NLX-112 in larger clinical trials.
Note: Further emerging therapies and their detailed assessment will be provided in the final report...
Parkinson's Disease Levodopa-induced Dyskinesia Drugs Market Insights
Commonly used drug classes in the treatment of Parkinson's disease (PD), including Levodopa-induced Dyskinesia (LID), encompasses a range of therapeutic options. Dopamine Agonists like pramipexole (Mirapex) and ropinirole (Requip) emulate dopamine's brain actions, often serving as supplementary or initial therapy in younger PD patients. By alleviating motor symptoms, they potentially delay LID onset compared to levodopa. Levodopa preparations, often combined with carbidopa (Sinemet), stand as the foremost treatment for managing PD's motor symptoms. However, their prolonged usage might provoke dyskinesias, including LID. Monoamine Oxidase-B (MAO-B) Inhibitors such as selegiline (Eldepryl, Zelapar) and rasagiline (Azilect) impede dopamine breakdown by inhibiting MAO-B, possibly extending dopamine's effects and decreasing LID risk. Catechol-o-methyltransferase (COMT) Inhibitors like entacapone (Comtan) and tolcapone (Tasmar) extend levodopa's effects by blocking COMT, which metabolizes levodopa peripherally. Typically adjunctive to levodopa, they manage motor fluctuations and may indirectly influence LID. Anticholinergics, though capable of reducing tremors and muscle rigidity in PD by modulating acetylcholine levels, are less commonly used due to cognitive decline and hallucination exacerbation risks. These drug classes offer diverse mechanisms to manage PD symptoms, including LID, with varying efficacy and tolerability among individuals.
Parkinson’s disease Levodopa-induced Dyskinesia Market Outlook
Therapeutic options for managing Parkinson’s disease Levodopa-induced Dyskinesia include non-pharmacological treatment, such as bandage contact lenses, topical ocular cycloplegic agents, topical ocular nonsteroidal anti-inflammatory agents (NSAIDs), and oral pharmaceutical agents. For treating patients with acute pain, there are generally two categories of oral medications: non-opioid medications and opioid medications. Non-opioid oral medications include prescription or OTC NSAIDs and acetaminophen. Pain associated with surgery, injury, infection, or inflammation at the front of the eye is typically treated with a topical steroid, topical NSAID, systemic NSAID, lubricant ointment, gel or drops, bandage contact lens, or a few doses of oral opiate or topical anesthetic.
US FDA approved GOCOVRI (amantadine) (2017) extended-release capsules as the first drug for treating PD patients receiving levodopa-based therapy. Later in 2021, the FDA also approved GOCOVRI extended-release tablets as an adjunctive treatment to levodopa/carbidopa in patients with PD experiencing off episodes. Although several drugs have been approved for treating PD patients, no drug except GOCOVRI is approved for Parkinson’s disease Levodopa-induced Dyskinesia. Nevertheless, continuous efforts are being made by pharma giants to bring novel and new therapies that can meet the needs of Parkinson’s disease Levodopa-induced Dyskinesia patients. Furthermore, several factors, including advancements in treatment options, increasing prevalence of Parkinson's disease, evolving regulatory landscapes, and growing awareness among healthcare professionals and patients will change the market dynamics for Parkinson’s disease Levodopa-induced Dyskinesia.
Several Parkinson's Disease Levodopa-induced Dyskinesia Companies are working robustly on many new therapies, including NLX-112 (Neurolixis SAS/Gala laboratories), JM-010 (Bukwang Pharmaceutical/Contera Pharma), KETARX (ketamine) (PharmaTher), AV-101 (Vistagen), and others anticipated to positively influence the market size Parkinson’s disease Levodopa-induced Dyskinesia during the forecast period [2024-2034].
- According to DelveInsight, the overall Parkinson's Disease Levodopa-induced Dyskinesia Market Dynamics are anticipated to change during the forecast period (2024-2034) owing to the expected launch of emerging therapies, increasing awareness among healthcare providers and patients, coupled with technological advancements in diagnostic techniques, and rising aging population in the 7MM.
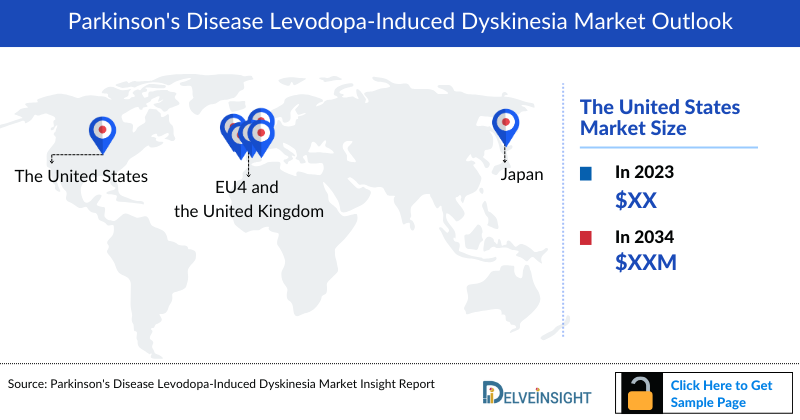
- In 2023, the total Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market Size in the US was approximately USD 900 thousand million.
- Among the EU4 and the UK, Germany holds the highest Parkinson's Disease Levodopa-induced Dyskinesia Market Size of around USD 135 thousand million followed by France, and Italy with approximately USD 115 thousand million, and USD 95 thousand million respectively. These numbers are expected to change during the forecast period (2024-2034) driven by the ongoing research and development efforts that may lead to the discovery of more effective medications and therapies for treating Parkinson’s disease Levodopa-induced Dyskinesia.
- Japan, accounted for nearly 6% total Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market Size in 2023.
Parkinson’s disease Levodopa-induced Dyskinesia Uptake
This section focuses on the uptake rate of potential Parkinson's Disease Levodopa-induced Dyskinesia drugs expected to be launched in the market during 2020–2034. For example, Neurolixis SAS/Gala laboratories NLX-112, a serotonin 5-HT1A receptor activator that exerts inhibitory effects, regulates neurotransmitter release and reduces dyskinesia symptoms by modulating the serotonergic system.
Parkinson’s disease Levodopa-induced Dyskinesia Pipeline Development Activities
The Parkinson's Disease Levodopa-induced Dyskinesia therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Parkinson's Disease Levodopa-induced Dyskinesia Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Parkinson's Disease Levodopa-induced Dyskinesia therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Parkinson’s disease Levodopa-induced Dyskinesia.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the Parkinson’s disease Levodopa-induced Dyskinesia evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the University of California, University of South Florida, Colorado State University, University of Birmingham, and Hospital Virgen De La Salud, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Parkinson’s disease Levodopa-induced Dyskinesia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
According to physicians, a primary challenge in Parkinson’s disease Levodopa-induced Dyskinesia treatment lies in managing the delicate balance between controlling motor symptoms and minimizing dyskinesias. While there are several medications and therapeutic approaches available for managing Parkinson’s disease Levodopa-induced Dyskinesia none are entirely effective for all patients, and the options for managing dyskinesias are somewhat limited. This highlights the need for continued research and development of novel therapies targeting dyskinesias specifically.
Furthermore, the response to levodopa and the development of dyskinesias vary widely among PD patients. Some individuals may experience dyskinesias early in the disease course, while others may develop them after years of levodopa therapy. This variability makes it challenging to predict and manage dyskinesias effectively.
Parkinson's Disease Levodopa-induced Dyskinesia Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Parkinson's Disease Levodopa-induced Dyskinesia treatment market landscape.
Attribute Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in Parkinson’s disease Levodopa-induced Dyskinesia trials, one of the most important primary outcome measures is complete eschar removal.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Parkinson's Disease Levodopa-induced Dyskinesia Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. Reimbursement challenges in accessing medical care and treatment for individuals with Parkinson’s disease Levodopa-induced Dyskinesia (PD-LID) can be significant, as specialized care often encompasses diagnosis, treatment, and ongoing management. Health insurance plans may offer limited coverage for certain medical treatments and therapies, particularly those specific to Parkinson’s disease Levodopa-induced Dyskinesia, leaving families with high out-of-pocket expenses. Additionally, accessing specialized healthcare providers with expertise in PD-LID care may be challenging, and insurance coverage may not always fully reimburse associated costs.
However, some companies provide assistance programs to alleviate financial burdens. For example, the GOCOVRI Co-pay Program offers eligible patients the opportunity to pay no more than USD 20 in copay or cost-sharing for each GOCOVRI prescription filled until reaching the maximum annual benefit. Patients may apply this assistance to up to two prescriptions of GOCOVRI per month, as needed.
Parkinson's Disease Levodopa-induced Dyskinesia Therapeutics Market Report Scope
- The Parkinson's Disease Levodopa-induced Dyskinesia therapeutics market report covers a segment of key events, an executive summary, descriptive overview of Parkinson’s disease Levodopa-induced Dyskinesia, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current treatment landscape.
- A detailed review of the Parkinson’s disease Levodopa-induced Dyskinesia market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Parkinson’s disease Levodopa-induced Dyskinesia market.
Parkinson’s disease Levodopa-induced Dyskinesia Therapeutics Market Report Insights
- Patient-based Parkinson's Disease Levodopa-induced Dyskinesia Market Forecasting
- Therapeutic Approaches
- Parkinson’s disease Levodopa-induced Dyskinesia Pipeline Drugs Analysis
- Parkinson’s disease Levodopa-induced Dyskinesia Market Size and Trends
- Existing and Future Parkinson's Disease Levodopa-induced Dyskinesia Drugs Market Opportunity
Parkinson’s disease Levodopa-induced Dyskinesia Therapeutics Market Report Key Strengths
- 11 years Parkinson's Disease Levodopa-induced Dyskinesia Market Forecast
- The 7MM Coverage
- Parkinson’s disease Levodopa-induced Dyskinesia Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Parkinson's Disease Levodopa-induced Dyskinesia Market Forecast Assumptions
Parkinson’s disease Levodopa-induced Dyskinesia Therapeutics Market Report Assessment
- Current Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market Practices
- Parkinson's Disease Levodopa-induced Dyskinesia Unmet Needs
- Parkinson's Disease Levodopa-induced Dyskinesia Pipeline Drugs Analysis Profiles
- Parkinson's Disease Levodopa-induced Dyskinesia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions
Parkinson's Disease Levodopa-induced Dyskinesia Therapeutics Market Insights
- What was the Parkinson’s disease Levodopa-induced Dyskinesia drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What was the total Parkinson's Disease Levodopa-induced Dyskinesia treatment market size by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will NLX-112, and JM-010 affect the treatment paradigm of Parkinson’s disease Levodopa-induced Dyskinesia?
- How will GOCOVRI compete with emerging products?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Parkinson's Disease Levodopa-induced Dyskinesia Epidemiology Insights
- What are the disease risks, burdens, and Parkinson's Disease Levodopa-induced Dyskinesia Unmet Needs? What will be the growth opportunities across the 7MM concerning the patient population with Parkinson’s disease Levodopa-induced Dyskinesia?
- What is the historical and forecasted Parkinson’s disease Levodopa-induced Dyskinesia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the above-mentioned countries, which country would have the highest diagnosed prevalent Parkinson’s disease Levodopa-induced Dyskinesia population during the forecast period (2023–2034)?
- What factors are factors contributing to the growth of Parkinson’s disease Levodopa-induced Dyskinesia cases?
Current Parkinson's Disease Levodopa-induced Dyskinesia Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Parkinson’s disease Levodopa-induced Dyskinesia? What are the current guidelines for treating Parkinson’s disease Levodopa-induced Dyskinesia in the US and Europe?
- How many companies are developing therapies for the treatment of Parkinson’s disease Levodopa-induced Dyskinesia?
- How many emerging therapies are in the mid-stage and late stage of development for treating Parkinson’s disease Levodopa-induced Dyskinesia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of approved therapies?
- What is the 7MM historical and forecasted Parkinson’s disease Levodopa-induced Dyskinesia Drugs Market?
Reasons to Buy
- The Parkinson's Disease Levodopa-induced Dyskinesia therapeutics market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Parkinson’s disease Levodopa-induced Dyskinesia drugs market.
- Insights on patient burden/disease Parkinson's Disease Levodopa-induced Dyskinesia prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Parkinson's Disease Levodopa-induced Dyskinesia drugs market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the Parkinson's Disease Levodopa-induced Dyskinesia Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies for Parkinson’s disease Levodopa-induced Dyskinesia, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Parkinson's Disease Levodopa-induced Dyskinesia drugs market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles