Peripheral Artery Disease Market
Key Highlights:-
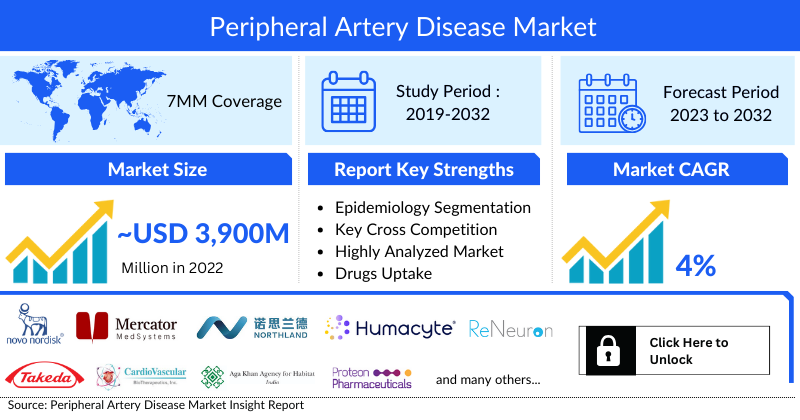
- In 2022, the market size of Peripheral Artery Disease was highest in the US among the 7MM accounting for approximately USD 4 billion, and lowest in Spain with USD 130 million which is further expected to increase by 2032.
- The majority of Peripheral Artery Disease patients were estimated in the US followed by EU4 and the UK and Japan.
- The total market size of the Peripheral Artery Disease treatment market is anticipated to experience growth during the forecast period (2023-2032) due to emerging treatment that includes ACP-01, Honedra, REX-001, AMG0001, and others.
- Gender-specific diagnosed prevalent cases of Peripheral Artery Disease were higher in females (9 million cases) as compared to males (8 million) in 2022 in the 7MM.
Request for unlocking the CAGR of Peripheral Artery Disease Market
DelveInsight's “Peripheral Artery Disease Market Insights, Epidemiology and Market Forecast– 2032” report delivers an in-depth understanding of the Peripheral Artery Disease, historical and forecasted epidemiology as well as the Peripheral Artery Disease market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Peripheral Artery Disease market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Peripheral Artery Disease market size from 2019 to 2032. The report also covers current Peripheral Artery Disease treatment practice/algorithm and Peripheral Artery Disease unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2019-2032
Peripheral Artery Market Disease: Treatment Algorithm and Disease Understanding
Peripheral Artery Disease Overview
Peripheral artery disease is a common manifestation of arterial stenosis of the lower and/or upper extremities that reduces arterial flow. It is well known that Peripheral Artery Disease is associated with elevated morbidity and mortality with cardiovascular (CV) disease. In Peripheral Artery Disease, the legs or arms—usually the legs—do not receive enough blood flow to keep up with the demand, which may cause leg pain when walking (claudication) and other symptoms.
Many people with Peripheral Artery Disease have no symptoms. The most common symptom of lower-extremity Peripheral Artery Disease is painful muscle cramping in the hips, thighs, or calves when walking, climbing stairs, or exercising. However, those who develop a painful ache in their legs when they walk usually disappear after a few minutes of rest. The medical term for this is “intermittent claudication”. The symptoms of Peripheral Artery Disease often develop slowly over time. If your symptoms develop quickly or get suddenly worse, it could be a sign of a serious problem requiring immediate treatment.
Peripheral Artery Disease Diagnosis
Physical examination of the legs for symptoms like shiny skin, brittle toenails, hair loss on legs and feet, and leg ulcers are evaluated for the diagnosis of Peripheral Artery Disease. The ankle-brachial pressure index (ABPI) test is widely used to diagnose Peripheral Artery Disease. Further testing, like an ultrasound scan or an angiogram, is done when there is uncertainty about the diagnosis.
A patient on suffering from atherosclerosis and affected by related risk factors experiences symptoms of Peripheral Artery Disease. To confirm the disease the patient goes to the doctor and get diagnosed with stage 0 to 6 as per Rutherford classification. Treatment according to stage is initiated. The condition may get worse affected by factors such as age, obesity, smoking, etc., and the patient is then diagnosed with further severe condition known as CLI. Further treatment is proceeded for related condition and stage. However when it comes to analyzing the real-world scenario in varying geographies, there are some differences in diagnostic criteria, and risk stratification that have been proposed by other organizations in certain European countries.
Further details related to country based variations are provided in the reported...
Peripheral Artery Disease Treatment
There is no cure for Peripheral Artery Disease, but lifestyle changes and medicine can help reduce the symptoms. Medications called statins are commonly prescribed. If Peripheral Artery Disease is so far progressed and does not respond to noninvasive therapies such as lifestyle changes, medication, or both, surgery may be necessary.
Medical therapy aims to reduce the risk of future cardiovascular morbidity and mortality in patients with high ischemic risk. Secondary prevention includes using antiplatelet agents and angiotensin-converting enzyme (ACE) inhibitors and managing other risk factors such as tobacco use, diabetes, low-density lipoprotein levels, and hypertension. Three main treatment options for improving functional status in patients with intermittent claudication are exercise training, medical therapy, and revascularization.
Many cell therapies are in development pipeline to target the population who are not suitable for revascularization or showed poor results after revascularization which might change the dynamics of current treatment market in 7MM countries.
Peripheral Artery Disease Epidemiology
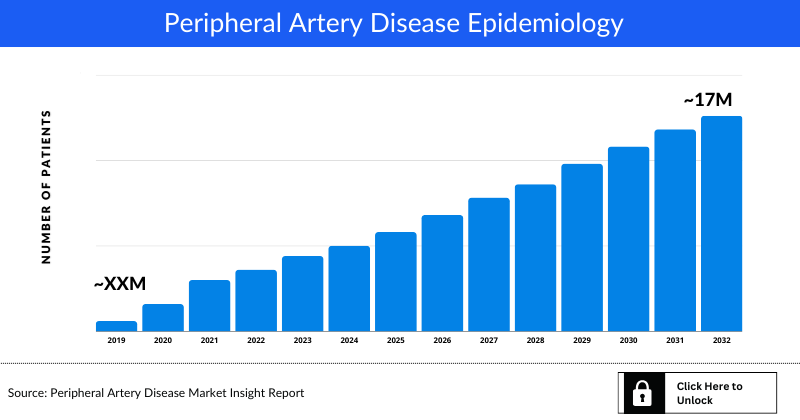
As the market is derived using patient based model, the Peripheral Artery Disease epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Total Diagnosed Prevalent Cases of Peripheral Artery Disease, Gender-specific Diagnosed Prevalent Cases of Peripheral Artery Disease, Age-specific Diagnosed Prevalent Cases of Peripheral Artery Disease, and Severity-specific Diagnosed Prevalent Cases of Peripheral Artery Disease in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), and the UK, and Japan from 2019 to 2032. The total prevalent cases of Peripheral Artery Disease in the 7MM comprised of approximately 17 million cases in 2022 and are projected to increase during the forecasted period (2023-2032)
- According to DelveInsight estimates, the US accounted for approximately 9 million cases, which was the highest diagnosed prevalent cases of Peripheral Artery Disease, followed by EU4 and the UK with 5 million cases, and Japan with 2 million cases in 2022. These cases are expected to increase in the US, EU4 and the UK, and Japan by 2032
- Among the European countries, Germany had the highest diagnosed prevalent population of Peripheral Artery Disease (approximately 2 million cases), followed by Italy (approximately 1 million cases) in 2022. On the other hand, Spain (0.7 million cases) had the lowest diagnosed prevalent population in EU4 and the UK countries and the 7MM.
- Peripheral Artery Disease has been identified as a male-dominant disease; however, in our analysis, the number of women suffering was higher than males. In 2022, 49% cases of Peripheral Artery Disease were of males, while 51% cases were of Females in the 7MM.
Peripheral Artery Disease Recent Developments
- In April 2025, Orchestra BioMed received FDA approval for an IDE amendment to initiate its Virtue® Sirolimus AngioInfusion Balloon™ (Virtue SAB) trial for coronary in-stent restenosis (ISR). The pivotal U.S. study will compare Virtue SAB with Boston Scientific’s AGENT™ paclitaxel-coated balloon.
Peripheral Artery Disease Drug Chapters
Drug chapter segment of the Peripheral Artery Disease report encloses the detailed analysis of Peripheral Artery Disease marketed drugs and late stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Peripheral Artery Disease clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Peripheral Artery Disease Marketed Drugs
Collategene (beperminogene perplasmid): AnGes, Inc/Mitsubishi Tanabe Pharma
Collategene (HGF plasmid) is a regenerative medical product, and its main component is a plasmid DNA encoding the HGF gene of 5181 base pairs, containing the cDNA for HGF (Beperminogene Perplasmid). Collategene administered by injection into muscles near the ischemic focus is expected to induce angiogenesis through HGF production/release and improve the ischemic state of the limb by increasing the number of blood vessels and blood flow.
In 2019, the Japanese government approved the country’s first gene therapeutic drug, Collategene, to treat critical limb ischemia; it was conditional, time-limited approval. The company is also targeting to enter the US market, which give competition to other emerging therapies.
Note: Detailed current therapies assessment will be provided in the full report of Peripheral Artery Disease
Peripheral Artery Disease Emerging Drugs
ACP-01: Hemostemix Inc.
ACP-01, Hemostemix’s lead clinical candidate, is an autologous cell therapy to treat critical limb ischemia (CLI) in patients facing amputation. ACP-01 consists of cells derived from the patient’s blood and modified with Hemostemix’s technology that forms new blood vessels.
ACP-01 is being developed as an autologous cell therapy for CLI, which means injecting the patient's cell population from peripheral blood to form new blood vessels and saving the limb. Hemostemix’s this drug is in Phase II safety clinical trial for critical limb ischemia. Although the trial status on CT is unknown, based on the positive interim results of Phase II, ACP-01 can get approval as the first commercialized autologously and allogeneically treating product.
Note: Detailed emerging therapies assessment will be provided in the final report.
Drug Class Insights
The existing Peripheral Artery Disease treatment is mainly dominated by Standard of Care for asymptomatic, symptomatic, intermittent claudication, and critical limb ischemia.
Peripheral Artery Disease treatment can be complicated and individualized, but the primary goal is to ease pain and restore blood flow to save the leg. The current treatment strategies include endovascular revascularization, surgical revascularization, and primary amputation, and endovascular revascularization is a fundamental strategy to limb preservation. Other than surgical approaches, therapeutic angiogenesis can be achieved by gene and cell therapy. It has raised hope for patients who cannot undergo standard revascularization treatment. Collategene (HGF plasmid) is the only gene therapy that received approval in Japan. The company is also targeting to enter the US market. Apart from this, many cell therapies are under development.
Peripheral Artery Disease Market Outlook
The overall medical treatment for Peripheral Artery Disease is comprehensive and involves lowering cholesterol, antiplatelet therapy, anticoagulation, peripheral vasodilators, blood pressure control, exercise therapy, and quitting smoking. Numerous medications are available for managing claudication symptoms, secondary prevention of cardiovascular problems, and limb salvage therapy. Lifestyle change, medication management, endovascular therapies, and surgical interventions are different treatment options.
Following this regime can lessen atherosclerosis-related systemic problems like stroke, myocardial infarction, and limb-related complications, including critical limb ischemia and amputation. Peripheral artery disease is undertreated compared to coronary artery disease.
The current treatment strategies include endovascular revascularization, surgical revascularization, and primary amputation, and endovascular revascularization is a fundamental strategy to limb preservation. Other than surgical approaches, therapeutic angiogenesis can be achieved by gene and cell therapy. Collategene (HGF plasmid) is the only gene therapy that received approval in Japan. Apart from this, many cell therapies are under development.
Several regenerative therapies, including angiogenic recombinant proteins, gene therapy, cell-based therapies (including stem or progenitor cells), and chemokines, have been tested in patients with Peripheral Artery Disease and non-revascularizable CLI and are under development which may further change the current market scenario.
Key players such as Ixaka Ltd (REX-001), Hemostemix (ACP-01), Lisata Therapeutics (Honedra), Reven Pharmaceuticals, Inc. (RJX), and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Peripheral Artery Disease.
- The total market size of Peripheral Artery Disease in the 7MM was approximately USD 3,930 million in 2022 and is projected to increase during the forecast period (2023-2032)
- The market size in the 7MM will increase at a CAGR of 4% due to increasing awareness of the disease and launch of the emerging therapy
- Among EU4 countries, Germany accounts for the maximum market size in 2022 while Spain occupies the bottom of the ladder in 2022
- In Japan, available medications include Collategene, Rivaroxaban, and others.
|
Report Metrics |
Details |
|
Study Period |
2019 to 2032 |
|
Base Year |
2021 |
|
Forecast Period |
2022 to 2032 |
|
CAGR | |
|
Peripheral Artery Disease Market Size |
USD 3,930 Million in 2022 |
|
Peripheral Artery Disease Key Companies |
NovoNordisk, Takeda, Mercator MedSystems, Inc., Beijing Northland Biotech. Co., Ltd., Ixaka Ltd, Humacyte, Inc., CardioVascular BioTherapeutics, Proteon Therapeutics, ReNeuron Limited, Alucent Biomedical, Athersys, ARCA biopharma, Ambulero, Venturis Therapeutics, and Many Others. |
Peripheral Artery Disease Drugs Uptake
This section focuses on the rate of uptake of the potential drugs expected to get launched in the market during the study period 2019-2032. For example, for REX-001, we expect the drug uptake to be medium with a probability-adjusted peak share of 2.0%, years to peak is expected to be 7 years from the year of launch.
Further detailed analysis of emerging therapies drug uptake in the report…
Peripheral Artery Disease Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Peripheral Artery Disease Clinical Trials Development Activities
The Peripheral Artery Disease clinical trials report covers the detailed information of collaborations, acquisition and merger, licensing and patent details for Peripheral Artery Disease emerging therapies.
KOL- Views
To keep up with current market trends, we take KOLs and SME's opinion working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Peripheral Artery Disease evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, drug uptake along with challenges related to accessibility, include Medical/scientific writers, Medical Professors, Director of Virginia University, Massachusetts General Hospital, Researchers from University of Palermo, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights, however interviews were conducted with 15+ KOLs in the 7MM. Centers such as West Virginia University, Gefässzentrum, Asklepios Westklinikum Hamburg, University of Palermo, etc. were contacted. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Peripheral Artery Disease market trend. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the Peripheral Artery Disease unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis, and Conjoint Analysis. In SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, and patient burden, and competitive landscape, cost-effectiveness and geographical accessibility of therapies are provided. These pointers are based on Analyst’s discretion and assessment of the patient burden, cost analysis and existing and evolving treatment landscape.
Conjoint Analysis is done to analyze multiple approved and emerging therapies on the basis of relevant attributes such as safety, efficacy, and frequency of administration, route of administration and order of entry. Scoring is given based on these parameters to analyze the effectiveness of a therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated, for instance, in Peripheral Artery Disease trials, one of the most important primary outcome measure is the change in total wound area of all ischemic ulcers.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability and the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Peripheral Artery Disease Market Access and Reimbursement
Critical limb ischemia is considered the most severe form of Peripheral Artery Disease and represents a significant financial burden to the healthcare system. The reported data suggest that for most patients with CLI, current Medicare reimbursement does not adequately cover the cost of providing care after open bypass surgery. As commercial insurers move toward Medicare reimbursement rates, more granular risk stratification profiles are needed to ensure open surgical care for patients with CLI remains financially sustainable.
The report further provides the detailed insights on the country wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out of pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs etc.
Peripheral Artery Disease Market Report Scope
- The report covers a segment of key events, executive summary, descriptive overview of Peripheral Artery Disease, explaining its causes, signs and symptoms, pathogenesis and currently available therapies
- Comprehensive insight has been provided into the epidemiology segments and forecasts, future growth potential of diagnosis rate, disease progression along with treatment guidelines
- Additionally, an all-inclusive account of both the current and emerging therapies along with the elaborative profiles of late-stage and prominent therapies, which will have an impact on at the current treatment landscape
- A detailed review of Peripheral Artery Disease market; historical and forecasted market size, market share by therapies, detailed assumptions and rationale behind our approach is included in the report, covering the 7MM drug outreach
- The patient-based peripheral artery disease market forecasting report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey and treatment preference that help in shaping and driving the 7MM Peripheral Artery Disease market
Peripheral Artery Disease Report Insights
- Patient-Based Peripheral Artery Disease Market Forecasting
- Peripheral Artery Disease Therapeutic Approaches
- Peripheral Artery Disease Pipeline Analysis
- Peripheral Artery Disease Market Size and Trends
- Existing and future Market Opportunity
Peripheral Artery Disease Report Key Strengths
- 10 Years Forecast
- 7MM Coverage
- Peripheral Artery Disease Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Peripheral Artery Disease Report Assessment
- Peripheral Artery Disease Current Treatment Practices
- Peripheral Artery Disease Unmet Needs
- Peripheral Artery Disease Pipeline Product Profiles
- Peripheral Artery Disease Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered In The Peripheral Artery Disease Market Report
Peripheral Artery Disease Market Insights:
- What was the Peripheral Artery Disease total market size, market size by therapies, market share (%) distribution in 2019 and how it would all look like in 2032? What are the contributing factors for this growth?
- How cell therapies are going to affect the treatment paradigm of Peripheral Artery Disease?
- What kind of uptake, ACP-01 is going to witness in the coming 10 years?
- How is AMG0001 going to compete with other treatment options?
- Which drug is going to be the largest contributor in 2032?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Peripheral Artery Disease Epidemiology Insights:
- What is the disease risk, burden and unmet needs of Peripheral Artery Disease? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Peripheral Artery Disease?
- What is the historical and forecasted Peripheral Artery Disease patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Why only limited patients appear with symptoms? Why is the current year diagnosis rate not high?
- What factors are affecting the increase in diagnosis of Peripheral Artery Disease cases?
Current Treatment Scenario, Marketed Drugs and Emerging Therapies:
- What are the current options for the treatment of Peripheral Artery Disease? What are the current treatment guidelines for the treatment of Peripheral Artery Disease in the US and Europe?
- How many companies are developing therapies for the treatment of Peripheral Artery Disease?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Peripheral Artery Disease?
- What are the recent novel therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Peripheral Artery Disease?
- What will be the impact of approved therapies expected patent expiry?
- What is the cost burden of approved therapies on patient?
- Patient acceptability in terms of preferred treatment options as per real world scenario?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted market of Peripheral Artery Disease?
Reasons to buy Peripheral Artery Disease Market Report
- The patient-based peripheral artery disease market forecasting report will help in developing business strategies by understanding latest trends and changing treatment dynamics driving the Peripheral Artery Disease Market
- Insights on patient burden/disease prevalence, evolution in diagnosis and factors contributing to the change in epidemiology of the disease during the forecast years
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under Conjoint analysis section to provide visibility around leading classes
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs
- To understand the perspective of Key Opinion Leaders’ around the accessibility, acceptability and compliance related challenges of existing treatment to overcome barriers in future
- Detailed insights on the Peripheral Artery Disease unmet need of existing market so that the upcoming players can strengthen their development and launch strategy



