Pirtobrutinib Market Summary
Key Factors Driving Pirtobrutinib Growth
- Market Share Gains and New Patient Starts
- Pirtobrutinib (Jaypirca), developed by Eli Lilly, is gaining traction in the B-cell malignancies market as a next-generation, non-covalent (reversible) BTK inhibitor.
- New patient starts are increasing, particularly among heavily pretreated patients with prior exposure to covalent BTK inhibitors, where treatment options have historically been limited.
- Lilly’s focused hematology-oncology commercial strategy, supported by strong physician education around resistance mechanisms, is driving early adoption in relapsed/refractory (R/R) settings.
- Expansion Across Key Indications
- Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma (CLL/SLL): Pirtobrutinib is positioned as a key therapy for patients who have progressed on or are intolerant to covalent BTK inhibitors, supported by durable response data.
- Mantle Cell Lymphoma (MCL): Initial approval and strong clinical activity in R/R MCL have established pirtobrutinib as an important option in later lines of therapy.
- Other B-Cell Malignancies: Ongoing studies are evaluating pirtobrutinib in marginal zone lymphoma, follicular lymphoma, and Waldenström’s macroglobulinemia.
- Earlier-Line and Combination Strategies: Pipeline development includes studies in earlier treatment lines and in combination with agents such as venetoclax and anti-CD20 antibodies, which could significantly expand market reach.
- Geographic Expansion
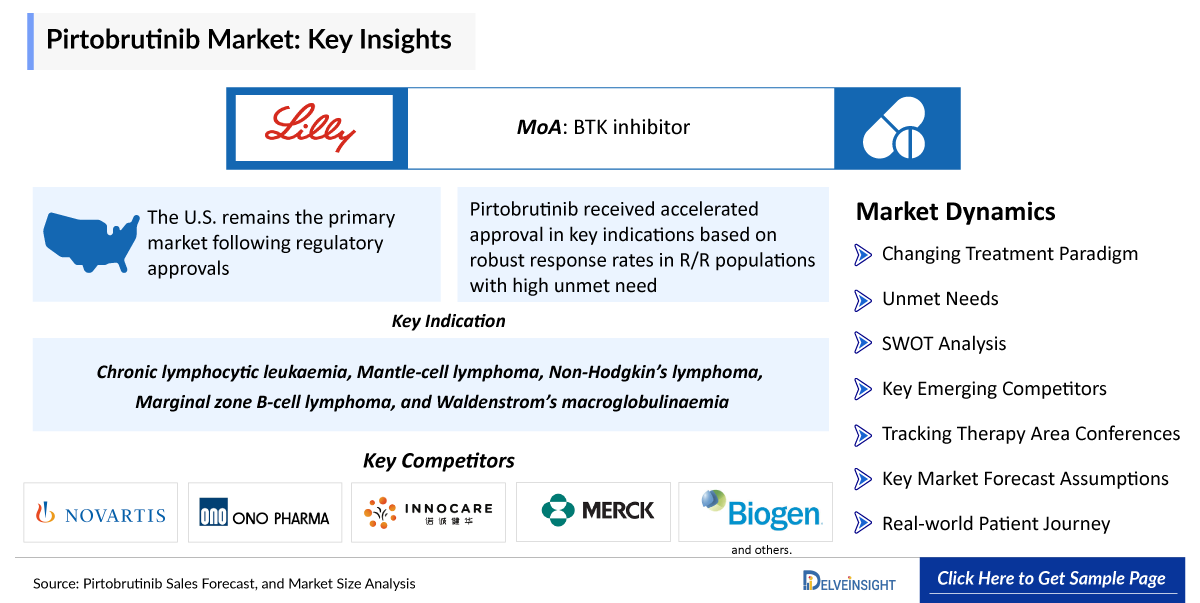
- United States as the Anchor Market: The U.S. remains the primary market following regulatory approvals, supported by strong uptake in academic and community oncology settings.
- Global Expansion Plans: Regulatory filings and clinical programs support expansion into Europe and other developed markets, where BTK inhibitor use is well established.
- Asia-Pacific Growth Potential: Rising diagnosis rates of hematologic malignancies and improved access to targeted therapies position Asia-Pacific as a long-term growth opportunity.
- New Indication Approvals
- Accelerated Approvals: Pirtobrutinib received accelerated approval in key indications based on robust response rates in R/R populations with high unmet need.
- Label Expansion Potential: Ongoing confirmatory trials are designed to support full approval and broaden indications into earlier lines of therapy.
- Revenue Diversification: Additional approvals across B-cell malignancies would significantly expand Lilly’s oncology portfolio and long-term revenue streams.
- Strong Hematologic Oncology Volume Momentum
- High Unmet Need in BTK-Exposed Patients: Patients who relapse after covalent BTK inhibitors represent a growing population with limited therapeutic alternatives.
- Compelling Clinical Profile: High overall response rates, activity across BTK resistance mutations (including C481), and favorable tolerability are driving sustained prescription momentum.
- Growing Real-World Utilization: Increasing real-world use in both academic and community settings is reinforcing physician confidence and supporting volume growth.
- Competitive Differentiation and Market Trends
- Non-Covalent BTK Inhibition: Pirtobrutinib’s reversible binding enables activity against BTK resistance mutations, differentiating it from first-generation and second-generation covalent BTK inhibitors.
- Favorable Safety and Tolerability: Lower rates of cardiac and bleeding-related adverse events relative to earlier BTK inhibitors improve suitability for long-term use.
- Shift Toward Precision Oncology: Increasing use of molecular profiling and resistance-driven treatment sequencing supports pirtobrutinib’s positioning.
- Growing Role of Real-World Evidence (RWE): RWE will be critical in reinforcing payer confidence, optimizing treatment sequencing, and supporting broader adoption.
Pirtobrutinib Recent Developments
In December 2025, Eli Lilly and Company announced that the US Food and Drug Administration (FDA) granted approval to Jaypirca (Pirtobrutinib, 100 mg & 50 mg tablets) for the treatment of adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) who have previously been treated with a covalent Bruton tyrosine kinase (BTK) inhibitor. This FDA action expands the Jaypirca label to include patients earlier in their treatment course and also converts the December 2023 accelerated approval for later-line CLL/SLL to a traditional approval.
“Pirtobrutinib Sales Forecast, and Market Size Analysis – 2034” report provides comprehensive insights of Pirtobrutinib for approved indication like Chronic lymphocytic leukaemia; Mantle-cell lymphoma; as well as potential indications like Non-Hodgkin's lymphoma; Marginal zone B-cell lymphoma; and Waldenstrom's macroglobulinaemia in the 7MM. A detailed picture of Pirtobrutinib’s existing usage in approved and anticipated entry and performance in potential indications in the 7MM, i.e., the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan for the study period 2020 –2034 is provided in this report along with a detailed description of the Pirtobrutinib for approved and potential indications. The Pirtobrutinib market report provides insights about Pirtobrutinib’s sales forecast, mechanism of action (MoA), dosage and administration, as well as research and development including regulatory milestones, along with other developmental activities. Further, it also consists of historical and current Pirtobrutinib performance, future market assessments inclusive of the Pirtobrutinib market forecast analysis for approved and potential indications in the 7MM, SWOT, analysts’ views, comprehensive overview of market competitors, and brief about other emerging therapies in respective indications. It also provides analysis of Pirtobrutinib sales forecasts, along with factors driving its market.
Pirtobrutinib Drug Summary
Pirtobrutinib (Jaypirca) is an oral, highly selective, non-covalent inhibitor of Bruton's tyrosine kinase (BTK) developed by Eli Lilly for relapsed or refractory B-cell malignancies. Unlike covalent BTK inhibitors such as ibrutinib or acalabrutinib that rely on binding to the C481 cysteine residue, pirtobrutinib reversibly binds to the ATP-binding pocket of BTK, maintaining potent inhibition even against C481 mutations, which are common resistance mechanisms, thereby blocking BCR signaling, B-cell proliferation, and survival in lymphomas. FDA-approved in 2023 for mantle cell lymphoma after ≥2 prior therapies and expanded in 2024 for chronic lymphocytic leukemia/small lymphocytic lymphoma after BTKi exposure, it offers once-daily dosing (200 mg) with a favorable profile, lower rates of cardiac toxicities like atrial fibrillation, and efficacy in heavily pretreated patients. The report provides Pirtobrutinib’s sales, growth barriers and drivers, post usage and approvals in multiple indications.
Scope of the Pirtobrutinib Market Report
The report provides insights into:
- A comprehensive product overview including the Pirtobrutinib MoA, description, dosage and administration, research and development activities in approved indications like Chronic lymphocytic leukaemia; Mantle-cell lymphoma; as well as potential indications like Non-Hodgkin’s lymphoma; Marginal zone B-cell lymphoma; and Waldenstrom’s macroglobulinaemia.
- Elaborated details on Pirtobrutinib regulatory milestones and other development activities have been provided in Pirtobrutinib market report.
- The report also highlights Pirtobrutinib‘s cost estimates and regional variations, reported and estimated sales performance, research and development activities in approved and potential indications across the United States, Europe, and Japan.
- The Pirtobrutinib market report also covers the patents information, generic entry and impact on cost cut.
- The Pirtobrutinib market report contains current and forecasted Pirtobrutinib sales for approved and potential indications till 2034.
- Comprehensive coverage of the late-stage emerging therapies for respective indications.
- The Pirtobrutinib market report also features the SWOT analysis with analyst views for Pirtobrutinib in approved and potential indications.
Methodology
The Pirtobrutinib market report is built using data and information sourced primarily from internal databases, primary and secondary research and in-house analysis by DelveInsight’s team of industry experts. Information and data from the secondary sources have been obtained from various printable and nonprintable sources like search engines, news websites, global regulatory authorities websites, trade journals, white papers, magazines, books, trade associations, industry associations, industry portals and access to available databases.
Pirtobrutinib Analytical Perspective by DelveInsight
In-depth Pirtobrutinib Market Assessment
- This Pirtobrutinib sales market forecast report provides a detailed market assessment of Pirtobrutinib for approved indication like Chronic lymphocytic leukaemia; Mantle-cell lymphoma; as well as potential indications like Non-Hodgkin’s lymphoma; Marginal zone B-cell lymphoma; and Waldenstrom’s macroglobulinaemia in the seven major markets, i.e., the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan. This segment of the report provides current and forecasted Pirtobrutinib sales data uptil 2034.
Pirtobrutinib Clinical Assessment
- The Pirtobrutinib market report provides the clinical trials information of Pirtobrutinib for approved and potential indications covering trial interventions, trial conditions, trial status, start and completion dates.
Pirtobrutinib Competitive Landscape
The report provides Insights on competitors and marketed products within the domain, along with a summary of emerging products and their respective launch dates, posing significant competition in the market.
Pirtobrutinib Market Potential & Revenue Forecast
- Projected market size for the Pirtobrutinib and its key indications
- Estimated Pirtobrutinib sales potential (Pirtobrutinib peak sales forecasts)
- Pirtobrutinib Pricing strategies and reimbursement landscape
Pirtobrutinib Competitive Intelligence
- Number of competing drugs in development (pipeline analysis)
- Pirtobrutinib Market positioning compared to existing treatments
- Pirtobrutinib Strengths & weaknesses relative to competitors
Pirtobrutinib Regulatory & Commercial Milestones
- Pirtobrutinib Key regulatory approvals & expected launch timelines
- Commercial partnerships, licensing deals, and M&A activity
Pirtobrutinib Clinical Differentiation
- Pirtobrutinib Efficacy & safety advantages over existing drugs
- Pirtobrutinib Unique selling points
Pirtobrutinib Market Report Highlights
- In the coming years, the Pirtobrutinib market scenario is set to change due to strong adoption, increased prescriptions and broader uptake in multiple immunological indications; which would expand the size of the market.
- The Pirtobrutinib companies are developing therapies that focus on novel approaches to treat/improve the disease condition, assess challenges, and seek opportunities that could influence Pirtobrutinib’s dominance.
- Other emerging products for Chronic lymphocytic leukaemia; Mantle-cell lymphoma; as well as potential indications like Non-Hodgkin’s lymphoma; Marginal zone B-cell lymphoma; and Waldenstrom’s macroglobulinaemia are expected to give tough market competition to Pirtobrutinib and launch of late-stage emerging therapies in the near future will significantly impact the market.
- A detailed description of regulatory milestones, and developmental activities, provide the current development scenario of Pirtobrutinib in approved and potential indications.
- Analyse Pirtobrutinib cost, pricing trends and market positioning to support strategic decision-making in the immunology landscape.
- Our in-depth analysis of the forecasted Pirtobrutinib sales data uptil 2034 will support the clients in decision-making process regarding their therapeutic portfolio by identifying the overall scenario of Pirtobrutinib in approved and potential indications.
Key Questions
- What is the class of therapy, route of administration and mechanism of action of Pirtobrutinib? How strong is Pirtobrutinib’s clinical and commercial performance?
- What is Pirtobrutinib’s clinical trial status in each individual indications such as Chronic lymphocytic leukaemia; Mantle-cell lymphoma; as well as potential indications like Non-Hodgkin’s lymphoma; Marginal zone B-cell lymphoma; and Waldenstrom’s macroglobulinaemia and study completion date?
- What are the key collaborations, mergers and acquisitions, licensing and other activities related to the Pirtobrutinib Manufacturers?
- What are the key designations that have been granted to Pirtobrutinib for approved and potential indications? How are they going to impact Pirtobrutinib’s penetration in various geographies?
- What is the current and forecasted Pirtobrutinib market scenario for approved and potential indications? What are the key assumptions behind the forecast?
- What are the current and forecasted sales of Pirtobrutinib in the seven major countries, including the United States, Europe (Germany, France, Italy, Spain) and the United Kingdom, and Japan?
- What are the other emerging products available and how are these giving competition to Pirtobrutinib for approved and potential indications?
- Which are the late-stage emerging therapies under development for the treatment of approved and potential indications?
- How cost-effective is Pirtobrutinib? What is the duration of therapy and what are the geographical variations in cost per patient?