Polymyalgia Rheumatica Market
- Polymyalgia rheumatica primarily affects older adults and shows a higher frequency in women.
- Chronic stiffness and pain often lead to poor sleep, fatigue, and mild depression, especially in untreated or poorly controlled cases.
- The precise cause remains undefined, involving genetic susceptibility (notably the HLA-DRB1*04 allele) and potential environmental triggers. Associations with infections like Mycoplasma pneumoniae or Parvovirus B19 are suggested but unproven.
- A paramount diagnostic challenge is evaluating for concomitant or evolving GCA. While temporal artery biopsy isn't routine for isolated polymyalgia rheumatica symptoms, red flags (new headache, vision/jaw symptoms, persistent high fever, refractory symptoms, or persistently high inflammatory markers) demand urgent assessment.
- Biologics like KEVZARA (sarilumab) are now approved for patients who are corticosteroid-resistant or intolerant.
- Several off-label therapies are used to manage symptoms or reduce long-term steroid exposure. These include disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, which is often prescribed to help control inflammation and lower the cumulative steroid dose.
- Advanced imaging like FDG-PET scans can detect vascular inflammation, even when traditional tests are inconclusive useful in steroid-resistant or atypical cases.
- The pipeline for polymyalgia rheumatica remains limited, with only a few companies—namely Sparrow Pharmaceuticals (SPI-47) and Novartis (secukinumab)—currently advancing therapeutic candidates for its treatment.
DelveInsight's “Polymyalgia Rheumatica– Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of polymyalgia rheumatica epidemiology, market, and clinical development in polymyalgia rheumatica. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the polymyalgia rheumatica market trends in the United States, EU4 (Germany, France, Italy, and Spain ), the United Kingdom, and Japan.
Polymyalgia rheumatica market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted polymyalgia rheumatica market size from 2020 to 2034 in 7MM. The report also covers current polymyalgia rheumatica treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan |
|
Polymyalgia Rheumatica Epidemiology |
Segmented by:
|
|
Polymyalgia Rheumatica Key companies |
|
|
Polymyalgia Rheumatica key therapies |
|
|
Polymyalgia Rheumatica Market |
Segmented by:
|
|
Analysis |
|
Polymyalgia Rheumatica Understanding and Treatment Algorithm
Polymyalgia Rheumatica Overview
Polymyalgia rheumatica manifests as rapid-onset, severe bilateral pain and stiffness primarily affecting the neck, shoulders, and hips. Most of the deterioration occurs in the morning, and the stiffness may persist for at least half an hour. Although this pain can be unbearable, it goes away with movement. The precise cause of polymyalgia rheumatica is unknown, however it primarily affects those over 50 and infrequently affects those under that age. Giant cell arteritis has a key connection to this illness. Many individuals who suffer from one of these disorders also exhibit signs of the other. Some cases steroids are being prescribed to heal the condition.
Polymyalgia Rheumatica Diagnosis
Healthcare professionals typically recommend physical examinations and laboratory tests to help identify the underlying cause of pain and stiffness. Since Polymyalgia rheumatica (PMR) can overlap with other conditions such as rheumatoid arthritis, spondyloarthritis, pseudogout, myositis, and others it's important to rule out these alternative diagnoses before confirming PMR.
Although there is no single definitive test for PMR, blood tests such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are commonly used to assess inflammation levels. Characteristic elevation of inflammatory markers, the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), is common.
In some cases, urine tests may be recommended to evaluate kidney function, especially to rule out other systemic conditions. Imaging techniques like X-rays and ultrasound scans may also be employed to examine joint and bone health, helping to exclude other musculoskeletal disorders.
Ultrasonography is pivotal, revealing hallmark features like subdeltoid bursitis, biceps tenosynovitis, or glenohumeral synovitis, integrated into classification criteria. Magnetic resonance imaging (MRI) offers superior sensitivity for detecting pelvic girdle involvement such as peri-tendinous enhancement or hip synovitis
Further details related to country-based variations in diagnosis are provided in the report.
Polymyalgia Rheumatica Treatment
Treatment for polymyalgia rheumatica typically begins with a low dose of corticosteroids, most commonly prednisone, which often provides rapid relief from pain and stiffness. If symptoms improve, the dosage is gradually reduced over the next 2 to 4 weeks, with adjustments guided by blood test results, such as ESR and CRP levels. Because long-term use of corticosteroids can cause serious side effects including weight gain, high blood pressure, and diabetes healthcare providers aim to use the lowest effective dose. In some cases, off-label medications may be prescribed to help reduce inflammation and minimize steroid exposure.
To support bone health during steroid treatment, calcium and vitamin D supplements are commonly recommended. Additionally, KEVZARA (sarilumab) is currently the only FDA-approved biologic specifically for PMR in patients who are resistant or intolerant to corticosteroids.
Finally, rest and regular exercise also play an important role in managing symptoms and maintaining mobility throughout the course of treatment.
Polymyalgia Rheumatica Epidemiology
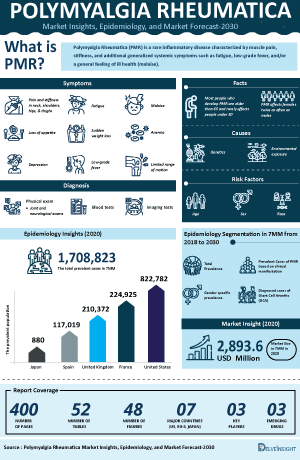
The polymyalgia rheumatica epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total prevalent cases of polymyalgia rheumatica, total diagnosed prevalent cases of polymyalgia rheumatica, gender-specific diagnosed prevalent cases of polymyalgia rheumatica, and treated cases of polymyalgia rheumatica in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- According to the analysis, the number of individuals living with polymyalgia rheumatica in the US is projected to grow during the forecast period (2025–2034). This rise stems from an aging population, increased life expectancy among older adults, and greater recognition of the condition in routine health assessments.
- According to the secondary research, the age-adjusted prevalence of polymyalgia rheumatica in the US is approximately 701 cases per 100,000 individuals aged 50 years and older.
- As per the secondary research, higher rates in females at 870 per 100,000 and in males at 508 per 100,000.
- As per the secondary research, approximately 15% of patients with PMR develop giant cell arteritis (GCA), and 40-50% of patients with GCA have associated PMR.
- The treated cases of polymyalgia rheumatica in the US are expected to increase, driven by demographic shifts toward an older population with greater disease susceptibility, improved diagnostic protocols that capture more cases, and expanded access to specialty services leading to higher rates of intervention.
Polymyalgia Rheumatica Drug Chapters
The drug chapter segment of the polymyalgia rheumatica report encloses a detailed analysis of polymyalgia rheumatica marketed drugs and emerging pipeline drugs. It also deep dives into polymyalgia rheumatica’s pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Products for Polymyalgia Rheumatica
KEVZARA (sarilumab): Regeneron Pharmaceuticals
KEVZARA (sarilumab) is a fully human monoclonal antibody developed by Regeneron and Sanofi that targets and inhibits both soluble and membrane-bound interleukin-6 receptors (IL‑6R). It is the first and only biologic approved by the FDA specifically for adults with polymyalgia rheumatica who are inadequate responders to corticosteroids or cannot tolerate tapering. By blocking IL‑6 signaling, KEVZARA significantly reduces inflammation and disease activity, enabling more patients to achieve steroid-free remission, with supportive evidence from the Phase III SAPHYR trial showing nearly threefold higher sustained remission rates compared to placebo
In February 2023, sarilumab has been granted FDA approval for treatment of polymyalgia rheumatica (PMR).
Emerging Products for Polymyalgia Rheumatica
SPI-47: Sparrow Pharmaceuticals
SPI-47, is an investigational oral treatment for polymyalgia rheumatica (PMR) that combines low-dose prednisolone with clofutriben (SPI-62), a targeted 11β-HSD1 inhibitor. This combination is intended to deliver the anti-inflammatory benefits of steroids while minimizing their long-term side effects by reducing cortisol activation in tissues such as bone, muscle, and liver. Currently in Phase IIa trials, SPI-47 is being studied as a potential steroid-sparing therapy to improve the safety and effectiveness of PMR treatment.
In June 2024, Sparrow Pharmaceuticals Presented data from ongoing Phase II Trial of Clofutriben (SPI-62) and Prednisolone as a treatment for polymyalgia rheumatica at EULAR 2024.
COSENTYX (secukinumab): Novartis
Secukinumab developed by Novartis is a fully human IgG1 monoclonal antibody that neutralizes interleukin‑17A (IL‑17A), a key cytokine driving inflammation. It is now being investigated as a treatment for polymyalgia rheumatica (PMR). In PMR, secukinumab is being studied for its potential to reduce inflammation and lower dependence on corticosteroids. A Phase III clinical trial (REPLENISH) is evaluating its effectiveness in achieving steroid-free remission in patients with relapsing PMR when combined with a short course of glucocorticoids.
|
Table 1: Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
SPI-47 |
Sparrow Pharmaceuticals |
IIa |
Polymyalgia Rheumatica |
SC |
HSD-1 inhibitor |
small molecule |
|
Secukinumab |
Novartis |
III |
Polymyalgia Rheumatica |
SC |
IL-17A inhibitor modulator |
monoclonal antibody |
Note: Detailed therapy assessment will be provided in the full report of polymyalgia rheumatica
Drug Class Insights
IL-17A inhibitor modulator
This class of drugs works by targeting and blocking interleukin‑17A (IL‑17A), a key cytokine involved in the body’s inflammatory response. These modulators usually monoclonal antibodies are specifically designed to bind to IL‑17A or its receptor, effectively inhibiting its activity and the resulting cascade of inflammation. By disrupting this interaction, IL‑17A inhibitors can significantly reduce the inflammation that contributes to the signs and symptoms of various inflammatory diseases. As such, they play a crucial role as targeted immunomodulators in managing chronic autoimmune and inflammatory conditions.
Interlukin-6 (IL-6)
Interleukin-6 (IL-6) inhibitors are a class of drugs that block the action of IL-6, a pro-inflammatory cytokine involved in the immune response and inflammation. In polymyalgia rheumatica (PMR), elevated IL-6 levels contribute to symptoms like muscle pain, stiffness, and fatigue. IL-6 inhibitors such as KEVZARA (sarilumab) and tocilizumab help reduce inflammation, relieve symptoms, and lower the need for long-term corticosteroid use, making them valuable as steroid-sparing therapies in patients with moderate to severe or relapsing PMR.
Polymyalgia rheumatica Market Outlook
Treatment options for polymyalgia rheumatica primarily focus on controlling inflammation and relieving symptoms, with low-dose corticosteroids, such as prednisone, being the standard and most effective first-line therapy. While newer insights have emerged such as the role of IL-6 in inflammation major therapeutic breakthroughs have been limited, and most treatment strategies still revolve around careful steroid tapering over months to years.
In patients who relapse frequently or are steroid-intolerant, off-label use of immunosuppressants like methotrexate or biologics such as KEVZARA (sarilumab) is considered.
These agents aim to maintain disease control while minimizing steroid exposure. Supportive measures, including calcium and vitamin D supplementation, weight-bearing exercise, and regular bone density monitoring, are essential to reduce steroid-related complications. Despite ongoing research, a definitive cure for PMR remains elusive, and treatment focuses on managing symptoms and preventing long-term harm.
Further, the pipeline of polymyalgia rheumatica is quite limited, with Sparrow Pharmaceuticals (SPI-47) and Novartis (secukinumab) currently in developing therapies for its treatment. These therapies have potential to transform polymyalgia rheumatica treatment landscape and market dynamic in upcoming years.
Polymyalgia Rheumatica drug uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034, which depends on the competitive landscape, safety, and efficacy data, along with the order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies' drug uptake in the report…
Polymyalgia Rheumatica Pipeline Development Activities
The report provides insights into different therapeutic candidates in the marketed and emerging stages. It also analyses key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for polymyalgia rheumatica therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Professors, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as University of Tennessee, Asahikawa Medical University Hospital, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or polymyalgia rheumatica market trends.
|
KOL Views |
|
“Polymyalgia rheumatica arises from a combination of genetic predisposition—most notably HLA-DR4—and environmental triggers, as suggested by seasonal patterns implicating pathogens such as parvovirus B19, Mycoplasma pneumoniae, and Chlamydia pneumoniae. Epigenetic modifications and dysregulated expression of cytokine-regulating genes further shape individual inflammatory phenotypes.” -Researcher, University of Tennessee, US |
|
“Polymyalgia rheumatica primarily affects older adults and shows a higher frequency in women. Commonly associated conditions include hypertension, diabetes mellitus, rheumatoid arthritis, and osteoporosis, while giant cell arteritis occurs infrequently. Broader analysis of diagnostic data highlights the need for continued investigation into the underlying clinical patterns and burden of disease.” - MD, Asahikawa Medical University Hospital, Japan
|
Qualitative Analysis
We perform qualitative and market intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyses multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyse the effectiveness of therapy.
The analyst analyses multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry.
In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, a descriptive overview of polymyalgia rheumatica, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborate profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the polymyalgia rheumatica market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Polymyalgia rheumatica market.
Polymyalgia rheumatica Report Insights
- Patient Population
- Therapeutic Approaches
- Polymyalgia rheumatica Pipeline Analysis
- Polymyalgia rheumatica Market Size and Trends
- Existing and Future Market Opportunity
Polymyalgia rheumatica Report Key Strengths
- Ten-Year Forecast
- 7MM Coverage
- Polymyalgia Rheumatica Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Polymyalgia rheumatica Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint)
FAQs
- What was the polymyalgia rheumatica total market size, the market size by therapies, market share (%) distribution in 2024, and what would it look like in 2034? What are the contributing factors for this growth?
- At what CAGR, the polymyalgia rheumatica market is expected to grow at the 7MM level during the study period (2020–2034)?
- How is the United States’ polymyalgia rheumatica competitive landscape evolving?
- How will upcoming emerging therapies will going to impact the current market share?
- What are the disease risks, burdens, and unmet needs of polymyalgia rheumatica?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to polymyalgia rheumatica?
- What is the historical and forecasted polymyalgia rheumatica patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- How are emerging therapies performing on parameters like efficacy, safety, route of administration (RoA), polymyalgia rheumatica treatment duration, and frequencies on the basis of their clinical trial results?
- What are the current options for the treatment of polymyalgia rheumatica? What are the current treatment guidelines for the treatment of polymyalgia rheumatica in the US and Europe?
- How many are emerging therapies in the mid and late-stage of development for polymyalgia rheumatica treatment?
- What key designations have been granted for the emerging therapies for polymyalgia rheumatica?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the polymyalgia rheumatica market.
- Insights on patient share/disease burden, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying upcoming players in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand KOLs’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.