Prosthetic Joint Infection Market
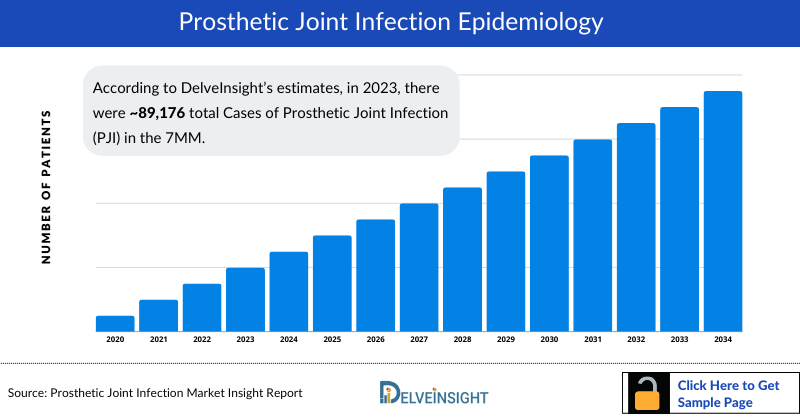
- According to DelveInsight’s estimates, in 2023, there were approximately 89,176 total Cases of Prosthetic Joint Infection (PJI) in the 7MM. Of these, the United States accounted for 63% of the Cases, while EU4 and the UK represented 34% and Japan accounted for nearly 3% of the Cases, respectively.
- The PJI market is set for steady growth, with a robust compound annual growth rate (CAGR) of around 5% anticipated from 2024 to 2034. This expansion in the 7MM is driven by the introduction of innovative therapies such as VT-X7, and PLG0206, along with the key drivers which include improved diagnostic techniques and advancements in antibiotic therapies.
- At present, there are no approved pharmacological therapies for treating PJI. Management primarily depends on off-label antibiotic use and surgical interventions, highlighting the need for regulatory-approved drugs to enhance outcomes and standardize treatment.
- A major barrier in Prosthetic Joint Infection treatment is the variability of infection-causing pathogens, which leads to inconsistent responses and is worsened by antibiotic resistance and a lack of targeted therapies. This complicates the development of standardized, effective treatment protocols.
- In August 2024, Osteal Therapeutics revealed encouraging results from two APEX randomized controlled trials of the VT-X7 KIT, demonstrating its potential as a major breakthrough in treating PJI.
DelveInsight’s “Prosthetic Joint Infection Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Prosthetic Joint Infection, historical and forecasted epidemiology, as well as the Prosthetic Joint Infection therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Prosthetic Joint Infection market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM PJI market size from 2020 to 2034. The report also covers PJI treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Prosthetic Joint Infection Market |
|
|
Prosthetic Joint Infections Market Size | |
|
Prosthetic Joint Infection Companies |
TenNor Therapeutics, Peptilogics, Osteal Therapeutics, Arrevus, and others. |
|
Prosthetic Joint Infection Epidemiology Segmentation |
|
Prosthetic Joint Infection Treatment Market
Prosthetic joint infection overview
PJI, also known as peri-prosthetic infection, is a severe complication following joint replacement surgery, significantly impacting individuals and healthcare systems. Characterized by localized pain, swelling, fever, and impaired joint function, PJI arises from a wide range of pathogens, predominantly Gram-positive bacteria like Staphylococci and Streptococci, but also Gram-negative bacteria, anaerobes, and fungi. The rising number of arthroplasties correlates with increased infection rates, necessitating early diagnosis and targeted interventions to mitigate its substantial clinical and economic burden.
Prosthetic joint infection diagnosis
The diagnosis of PJI requires a multifaceted approach, utilizing both clinical and laboratory methods to assess the host's response to infection. Peripheral blood tests, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin levels, provide valuable inflammatory markers, while synovial fluid analysis, particularly leukocyte count and granulocyte percentage, delivers a rapid and accurate distinction between PJI and aseptic failure.
Advanced imaging techniques, including CT, MRI, and three-phase bone scintigraphy, complement diagnostic efforts but may be limited by artifacts from metal prostheses. Emerging modalities like FDG-PET and the Alpha Defensin Lateral Flow test offer enhanced precision.
Additionally, sonication of removed prosthetic components increases sensitivity for detecting biofilm-related infections, and molecular diagnostics, particularly PCR, hold potential for rapid pathogen identification, although contamination risks must be managed. This comprehensive diagnostic algorithm ensures early and accurate identification of PJI, optimizing treatment outcomes.
Further details related to country-based variations are provided in the report…
Prosthetic joint infection treatment
The treatment of PJI necessitates a multifaceted approach combining surgical and antimicrobial strategies tailored to the specific pathogens involved. Surgical interventions may include open or arthroscopic debridement, prosthesis resection, or, in some cases, revision surgery, ideally conducted alongside pathogen-directed antimicrobial therapy. Antimicrobial regimens prioritize the use of narrow-spectrum antibiotics, such as cefazolin, rifampicin, and vancomycin, while companion drugs are essential for preventing resistance, particularly with Staphylococcal infections.
Emerging therapies, including antimicrobial peptides and biofilm disruption strategies, show promise but remain largely in preclinical stages. Continued research is essential to refine diagnostic accuracy and develop innovative treatment modalities, such as targeted implant coatings and localized antibiotic delivery systems, to enhance therapeutic efficacy and minimize the recurrence of PJI.
Prosthetic Joint Infection Epidemiology
As the market is derived using a patient-based model, the Prosthetic Joint Infection epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total number of arthroplasty cases, total Cases of PJI, gender-specific cases of PJI, and causative microorganism-specific cases of PJI in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- According to DelveInsight’s epidemiology model, in the 7MM, the total cases of PJI were approximately 89,176 in 2023. This number is anticipated to rise during the forecast period (2024-2034), driven by the aging population, along with increased joint replacement surgeries.
- In 2023, the US reported the highest number of PJI cases, totaling around 56,077, whereas Japan reported the fewest, with approximately 2,741 cases.
- Among EU4 and the UK, Germany reported the highest number of PJI cases in 2023, with approximately 13,469 cases. Italy followed with around 5,355 cases, and the UK had nearly 4,599 cases.
- In Japan in 2023, gender-specific cases of PJI included approximately 1,491 cases in males and 1,250 cases in females.
- In 2023, Japan recorded approximately 1,919 cases of PJI caused by gram-positive bacteria, compared to 699 cases attributed to gram-negative bacteria.
Prosthetic Joint Infection Drug Chapters
Prosthetic Joint Infection Emerging Drugs
VT-X7 (vancomycin hydrochloride and tobramycin sulfate): Osteal Therapeutics
VT-X7 is an advanced drug/device combination product developed by Osteal Therapeutics, specifically designed to deliver therapeutic concentrations of vancomycin hydrochloride and tobramycin sulfate directly to the joint space for the treatment of PJI.
This innovative seven-day therapy addresses the critical need for rapid and effective treatment of PJI, demonstrating significant clinical efficacy in a Phase II study, where 100% of patients received a new permanent joint prosthesis within seven days, with 93% remaining infection-free at one year. The US FDA has recognized VT-X7's potential by granting it Breakthrough Therapy Designation, alongside orphan drug, fast track, priority review, and Qualified Infectious Disease Product designations.
This accelerated path aims to enhance the commercialization of VT-X7, which shows promise in significantly improving current treatment standards for serious PJI conditions.
In August 2024, Osteal Therapeutics announced positive results from the two APEX (Abbreviated Protocol for Two Stage Exchange) randomized controlled clinical trials of the VT-X7 KIT for patients with PJI.
PLG0206: Peptilogics
PLG0206 is an innovative investigational therapeutic peptide developed by Peptilogics for the treatment of PJI. With a unique mechanism of action that disrupts bacterial membranes, PLG0206 effectively targets persistent pathogens within biofilms that evade standard antibiotic treatments. The US FDA has granted PLG0206 Orphan Drug Designation, Qualified Infectious Disease Product (QIDP) status, and Fast Track Designation, underscoring its therapeutic potential.
Recent interim data from the ongoing Phase Ib trial demonstrated a favorable safety profile, with no treatment-related serious adverse events reported and a low recurrence rate of 7% in the Low Dose cohort at Day 180, while the High Dose cohort showed no recurrences. These findings highlight PLG0206's promising role in addressing the significant clinical challenge posed by PJI.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
VT-X7 |
Antibacterials |
IV infusion at the local site |
Osteal Therapeutics |
II | |
|
PLG0206 |
Cell membrane permeability enhancers |
IV infusion at the local site |
Peptilogics |
Ib | |
|
XX |
XX |
XX |
XXX |
X |
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Drug Class Insights
The treatment of PJI primarily involves several drug classes, each playing a critical role in managing this complex condition. Broad-spectrum antibiotics, such as beta-lactams (e.g., cefazolin) and glycopeptides (e.g., vancomycin), are essential for targeting the diverse range of bacteria implicated in PJI, particularly gram-positive pathogens. Additionally, aminoglycosides (e.g., tobramycin) may be employed for their synergistic effects, especially in polymicrobial infections.
For biofilm-related infections, rifampicin is frequently used due to its ability to penetrate and disrupt bacterial biofilms. Furthermore, emerging therapies, such as antimicrobial peptides (e.g., PLG0206), represent novel approaches aimed at effectively addressing persistent infections by targeting bacterial membranes. The strategic combination of these drug classes enhances treatment efficacy and reduces the risk of recurrence, highlighting the importance of individualized antimicrobial regimens based on pathogen susceptibility profiles.
Continued in report…
Prosthetic Joint Infection Market Outlook
The treatment landscape for PJI necessitates a multifaceted approach combining medical and surgical strategies, driven by the imperative for individualized and effective antimicrobial therapies. Current regimens primarily involve well-established antibiotics such as cefazolin, rifampicin, and vancomycin, which must be tailored based on pathogen susceptibility profiles. Notably, rifampicin's efficacy against Staphylococcal biofilms underscores the need for combination therapy to mitigate rapid resistance development.
The emerging use of antimicrobial peptides, including investigational agents like PLG0206, presents a promising avenue for disrupting biofilms and combating persistent infections. Additionally, the VT-X7 irrigation system, delivering therapeutic concentrations of vancomycin and tobramycin directly to the joint space, exemplifies innovative drug/device combination approaches aimed at enhancing treatment outcomes.
Despite advances, the management of PJI remains challenging due to diagnostic complexities and the necessity for interdisciplinary collaboration. The future of PJI treatment will likely hinge on the integration of novel therapeutic modalities, improved diagnostic methods, and ongoing research to address the unmet needs in this field.
Continued in report…
Prosthetic Joint Infection Drugs Uptake
This section focuses on the uptake rate of potential Prosthetic Joint Infection drugs expected to be launched in the market during 2020–2034.
Further detailed analysis of emerging therapies drug uptake in the report…
Prosthetic Joint Infection Pipeline Development Activities
The report provides insights into Prosthetic Joint Infection clinical trials within in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for PJI.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on PJI's evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as the Hospital for Special Surgery in the US, the Autonomous University of Madrid in Spain, and Wansbeck General Hospital in the UK, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or PJI market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per the KOLs from the US, implant fixation can be augmented when necessary, most often in knee replacement using polymethylmethacrylate (PMMA) cement, which can also be loaded with antibiotic powder that elutes into the joint space.
As per the KOLs from the UK, traditionally, IV antibiotics have been used to treat patients with PJI, but to do that requires either a hospital stay or stringent at-home care. About 15–20 years ago, oral antibiotic usage emerged in Europe. European surgeons think it is a more modern, effective, and easier protocol.
As per the KOLs from Japan, patients receiving disease-modifying agents for inflammatory conditions or other immune-modulating therapies should manage their medications according to established guidelines, often in collaboration with their rheumatologist.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
DelveInsight’s ‘Prosthetic Joint Infection – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of prosthetic joint infection.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Scope of the Prosthetic Joint Infection Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of PJI explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the PJI market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM PJI market.
Prosthetic joint infection report insights
- Prosthetic Joint Infection Patient Population
- Prosthetic Joint Infection Therapeutic Approaches
- Prosthetic Joint Infection Pipeline Analysis
- Prosthetic Joint Infection Market Size and Trends
- Existing and Future Market Opportunity
Prosthetic joint infection report key strengths
- 11 years Forecast
- The 7MM Coverage
- Prosthetic Joint Infection Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Prosthetic Joint Infection Drugs Uptake
- Key Prosthetic Joint Infection Market Forecast Assumptions
Prosthetic joint infection report assessment
- Current Prosthetic Joint Infection Treatment Practices
- Prosthetic Joint Infection Unmet Needs
- Prosthetic Joint Infection Pipeline Product Profiles
- Prosthetic Joint Infection Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
- Prosthetic Joint Infection Market Drivers
- Prosthetic Joint Infection Market Barriers
Key Questions Answered In The Prosthetic Joint Infection Market Report
Prosthetic Joint Infection Market Insights
- What was the total market size of PJI, the market size of PJI by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will PLG0206 affect the treatment paradigm of PJI?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Prosthetic Joint Infection Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of PJI? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to PJI?
- What is the historical and forecasted PJI patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest prevalent PJI population during the forecast period (2024–2034)?
- What factors are contributing to the growth of PJI Cases?
Current Prosthetic Joint Infection Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of PJI? What are the current clinical and treatment guidelines for treating PJI?
- How many companies are developing therapies for the treatment of PJI?
- How many emerging therapies are in the mid-stage and late stage of development for treating PJI?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of PJI?
Reasons to Buy Prosthetic Joint Infection Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the PJI market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for PJI, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.