Psychosis in Parkinson’s and Alzheimer’s Disease Market
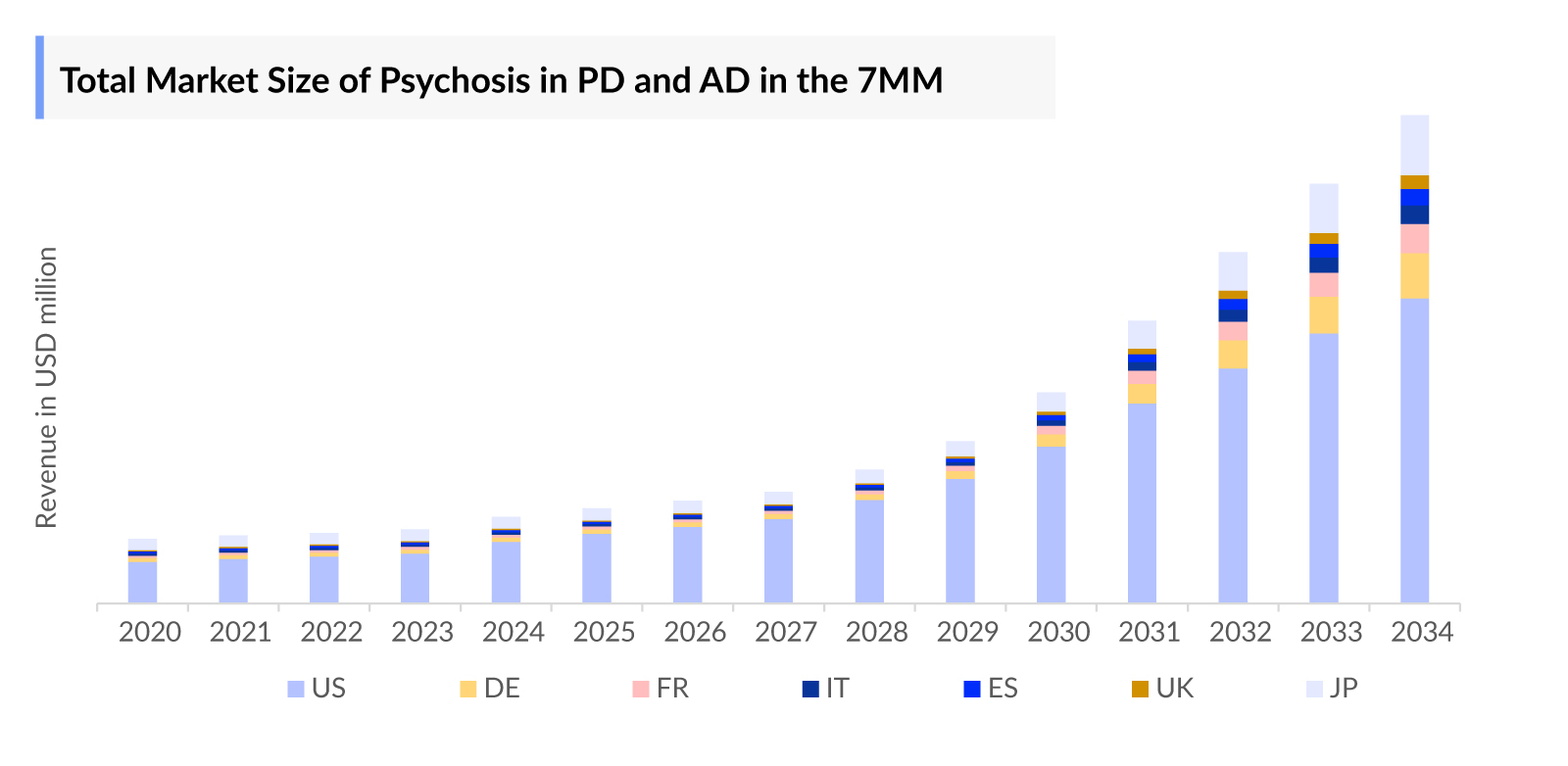
- According to DelveInsight’s analysis, the psychosis in Parkinson’s disease and Alzheimer’s disease market in the 7MM was valued at approximately USD 1,290 million in 2023 and is projected to grow over the forecast period from 2024 to 2034.
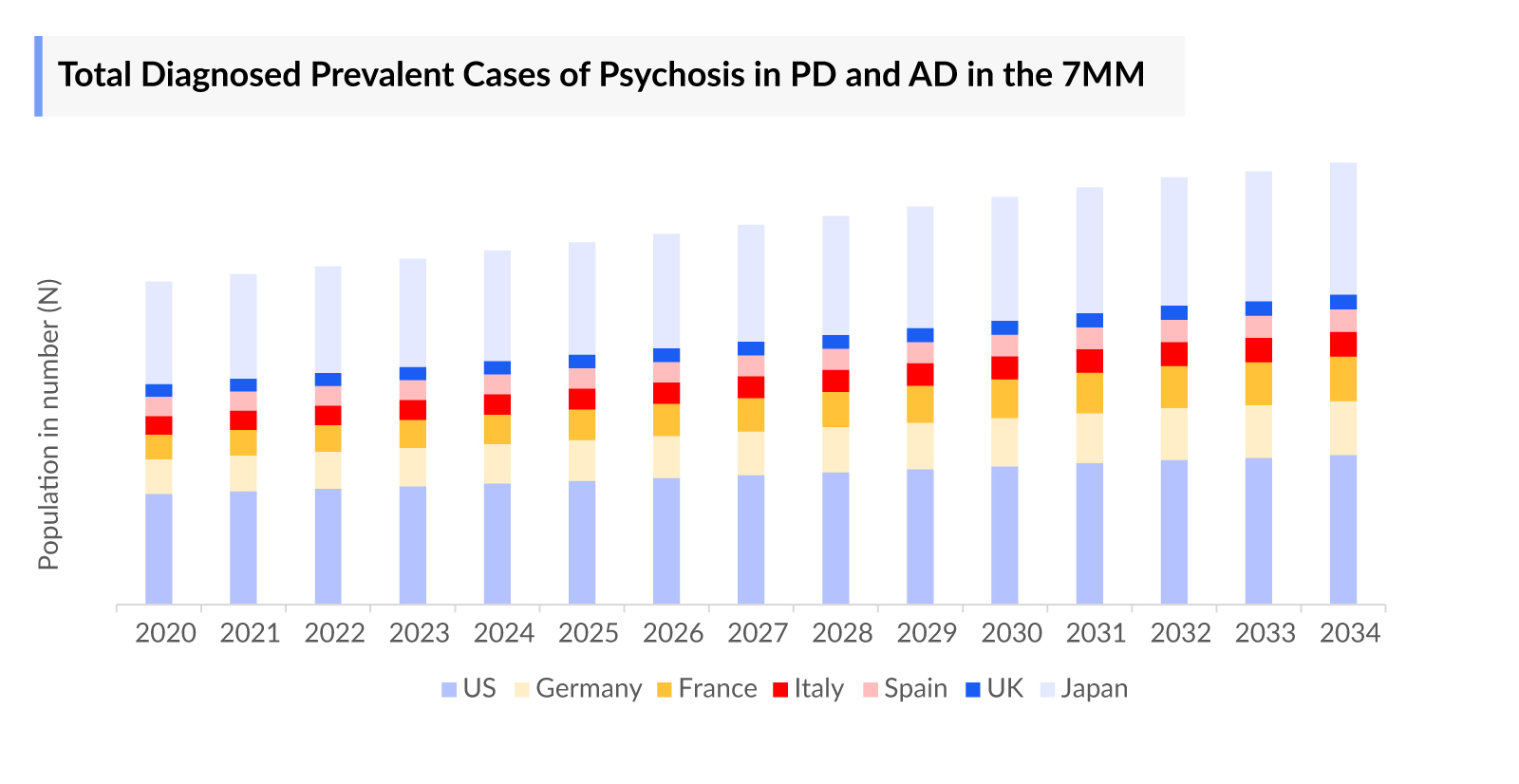
- According to DelveInsight’s estimates, in 2023, there were approximately 6.9 million diagnosed prevalent cases of psychosis in Parkinson’s disease and Alzheimer’s disease in the 7MM. Of these, the US accounted for 34% of the cases, while Japan represented nearly 31% and Germany with 11% of the cases, respectively.
- The psychosis in Parkinson’s’ disease and Alzheimer’s disease market is set for steady growth, driven by the introduction of innovative therapies such as Karuna Therapeutics' KarXT (xanomeline-trospium), Sunovion Pharmaceuticals' Ulotaront (SEP-363856), and Intra-Cellular Therapies' ITI-1284, among others.
- As the population ages, the occurrence of Parkinson’s disease and Alzheimer’s disease is expected to increase, driving a greater demand for effective psychosis treatments. This trend is contributing to a more comprehensive and patient-centered approach to managing psychosis in neurodegenerative diseases globally. Moreover, the increased awareness of psychosis as a significant aspect of Parkinson’s disease and Alzheimer’s disease might lead to better diagnosis and treatment options, contributing to market expansion.
- Key unmet needs in managing psychosis in Parkinson's and Alzheimer's disease include the absence of curative therapies and reliable biomarkers. Current screening tools are often cumbersome, making timely diagnosis and intervention challenging. While NUPLAZID is approved for treating psychosis in Parkinson's disease, there is no equivalent approved treatment for psychosis in Alzheimer's disease, highlighting a significant gap in care. Moreover, psychosis symptoms are often underreported or misattributed to other conditions, leading to a lack of accurate epidemiological data.
DelveInsight’s “Psychosis in Parkinson’s disease and Alzheimer’s disease Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of psychosis in Parkinson’s disease and Alzheimer’s disease, historical and forecasted epidemiology, as well as the psychosis in Parkinson’s disease and Alzheimer’s disease market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The psychosis in Parkinson’s disease and Alzheimer’s disease market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM psychosis in Parkinson’s disease and Alzheimer’s disease market size from 2020 to 2034. The report also covers psychosis in Parkinson’s disease and Alzheimer’s disease treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
Psychosis in Parkinson’s disease and Alzheimer’s disease Epidemiology |
|
|
Psychosis in Parkinson’s disease and Alzheimer’s disease Market |
|
|
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Analysis |
|
|
Psychosis in Parkinson’s Disease and Alzheimer’s Disease Market players |
|
|
Future opportunity |
Research into the underlying mechanisms of psychosis could lead to targeted therapies, particularly those focusing on the 5-hydroxytryptamine subtype 2A receptor, which shows promise in managing psychotic symptoms in Parkinson’s and Alzheimer’s disease. Improved access to care and advancements in personalized medicine may further expand opportunities for targeted treatments, driving market growth. |
Psychosis in Parkinson’s disease and Alzheimer’s disease Treatment Market
Psychosis in Parkinson’s disease and Alzheimer’s disease overview
According to the National Institute of Mental Health (NIMH), psychosis refers to a collection of symptoms that affect the mind, where there has been some loss of contact with reality. During an episode of psychosis, individuals’ thoughts and perceptions are disrupted, which leads to difficulty recognizing what is real and what is not.
Psychosis can be categorized into three broad groups: idiopathic psychosis, psychosis due to medical conditions (including neurodegenerative disorders), and toxic psychosis (due to substances of abuse, prescribed medications, or toxins). It is a syndrome embedded in several disorders, including Alzheimer’s disease and Parkinson’s disease, with severe psychotic features. Parkinson’s disease psychosis is a common phenomenon in Parkinson's disease patients associated with high morbidity and mortality, including decreased health-promoting behaviors and longer nursing home stays. It is characterized by visual hallucinations and other psychotic symptoms, including auditory hallucinations, depression, anxiety, delusions, or illusions that impact the longevity and quality of life. Alzheimer’s disease psychosis, a typical occurrence in AD patients, is linked to dementia and auditory and visual hallucinations. There is a gradual onset of multiple cognitive deficits with continuing cognitive decline.
Psychosis in Parkinson’s disease and Alzheimer’s disease diagnosis
Psychosis in Parkinson's disease and Alzheimer's disease is characterized by hallucinations and delusions, significantly impacting patients' quality of life. Diagnosis relies on clinical evaluation, often using caregiver reports. In Parkinson's disease, psychosis can arise from disease progression or medication effects, while in Alzheimer's disease, it typically correlates with cognitive decline.
Further details related to country-based variations are provided in the report…
Psychosis in Parkinson’s disease and Alzheimer’s disease treatment
The primary aim of the treatment is to reduce the frequency and severity of psychotic symptoms with minimal worsening of motor symptoms. Non-pharmacological approaches such as reality orientation, cognitive stimulation therapy (CST), reminiscence therapy, and psychodynamic therapy are preferred as an initial approach before or at least concurrent with medication in patients.
Atypical antipsychotics such as clozapine, quetiapine, olanzapine, risperidone, and aripiprazole are often considered in the initial therapy, which reduces the risk of later deterioration in patients. Of these, clozapine is well-studied and is considered the “gold standard” treatment in psychosis of PD; however, in older patients, it causes sedation and postural hypotension. Quetiapine is favored by many psychiatrists because of its better side effect profile. Apart from atypical antipsychotics, 5-HT3 receptor antagonists, acetylcholinesterase inhibitors, or other antidepressants are also used.
So far, the only FDA-approved medication includes Acadia’s NUPLAZID (pimavanserin), an atypical antipsychotic based on the principle of selective 5-HT2A inverse agonist with no dopaminergic, histaminergic, or muscarinic binding.
Read Insights on What Lies Ahead For Parkinson’s Disease Psychosis Treatment Market?
Psychosis in Parkinson’s disease and Alzheimer’s disease Epidemiology
As the Psychosis in Parkinson’s disease and Alzheimer’s disease market is derived using a patient-based model, the Psychosis in Parkinson’s disease and Alzheimer’s disease epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by diagnosed prevalent cases of Parkinson’s disease, diagnosed prevalent cases of Alzheimer's, gender-specific cases of Parkinson’s disease, gender-specific cases of disease Alzheimer's disease, age-specific cases of Parkinson’s disease, age-specific cases of Alzheimer's disease, diagnosed prevalent cases of psychosis in Parkinson’s disease, diagnosed prevalent cases of psychosis in Alzheimer's disease in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- The total diagnosed prevalent cases of Parkinson’s disease in the 7MM were estimated to be 2.6 million in 2023. The US accounted for approximately 45% of the total diagnosed prevalent cases of Parkinson’s disease, while EU4 and the UK accounted for 45%, and Japan for 10% in 2023. These cases are expected to increase by 2034.
- Among the EU4 and the UK, Germany accounted for the highest number of diagnosed prevalent cases of Parkinson’s disease i.e. 487 thousand, followed by France (223 thousand), while Spain accounted for the lowest number of cases i.e. 150 thousand in 2023.
- In 2023, the total number of diagnosed prevalent cases of Alzheimer's disease in the US accounted for 6.3 million, these cases are expected to change during the forecast period.
- According to estimates based on DelveInsight’s epidemiology model, the age-specific cases of Parkinson's disease in EU4 and the UK in 2023 were, approximately 18 thousand, 62 thousand, 338 thousand, and 773 thousand in the age group <50, 50–59, 60–69, and ≥70 years, respectively. Due to the increase in the overall diagnosed prevalent cases of Parkinson's disease, age-specific cases are expected to increase by 2034.
- According to estimates, females made up about 65% of the total diagnosed cases of Alzheimer’s disease in the US in 2023, while males accounted for the remaining 35%.
- According to DelveInsight analysis, there were 2.1 million diagnosed prevalent cases of Alzheimer's disease psychosis in Japan in 2023, with this number expected to grow at a CAGR of 1.9% by 2034. In contrast, the diagnosed prevalent cases of Parkinson's disease psychosis were reported at 37 thousand in 2023.
Psychosis in Parkinson’s disease and Alzheimer’s disease Drug Chapters
The drug chapter segment of the psychosis in Parkinson’s disease and Alzheimer’s disease market report encloses a detailed analysis of psychosis in Parkinson’s disease and Alzheimer’s disease-marketed drugs and mid to late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the psychosis in Parkinson’s disease and Alzheimer’s disease clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Marketed Psychosis in Parkinson’s disease and Alzheimer’s disease Drugs
NUPLAZID (pimavanserin): Acadia Pharmaceuticals
NUPLAZID is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. NUPLAZID is a non-dopaminergic, selective serotonin inverse agonist preferentially targeting 5-HT2A receptors that are thought to play an important role in Parkinson’s disease psychosis. ACADIA discovered this new chemical entity and holds worldwide rights to develop and commercialize NUPLAZID. The US FDA granted BTD designation to NUPLAZID (pimavanserin) for the treatment of Parkinson’s disease psychosis. In April 2016, the US FDA approved NUPLAZID (pimavanserin) for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis.
Note: Further marketed drugs and their details will be provided in the report…
Emerging Psychosis in Parkinson’s disease and Alzheimer’s disease Drugs
Ulotaront (SEP-363856): Sunovion Pharmaceuticals
Ulotaront, being developed by Sunovion Pharmaceuticals and its parent company Sumitomo, is a trace-amine associated receptor 1 (TAAR1) agonist with serotonin 1A receptor agonist activity. TAAR1 is a G-protein-coupled receptor (GPCR) that is expressed in cortical, limbic, and midbrain monoaminergic regions. It is activated by endogenous trace amines and is believed to play an important role in modulating dopaminergic, serotonergic, and glutamatergic circuitry.
Sunovion discovered ulotaront in collaboration with PsychoGenics based in part on a mechanism-independent approach using the in vivo phenotypic SmartCube platform and associated artificial intelligence algorithms. It is being jointly developed and commercialized by Otsuka, Sunovion, and Sumitomo Pharma.
Ulotaront (SEP-363856) has completed Phase II trial for the treatment of Parkinson’s disease psychosis, however further update is awaited. It is also being developed for the treatment of schizophrenia, adjunctive major depressive disorder, and generalized anxiety disorder.
KarXT (xanomeline-trospium): Karuna Therapeutics
KarXT (xanomeline-trospium), developed by Karuna Therapeutics, is an oral, investigational M1/M4-preferring muscarinic agonist for treating psychiatric and neurological conditions, including schizophrenia and psychosis in Alzheimer's disease.
KarXT combines xanomeline, a novel muscarinic agonist, with trospium, an approved muscarinic antagonist that does not measurably cross the blood-brain barrier, confining its effects to peripheral tissues, to preferentially stimulate muscarinic receptors in the CNS and unlock the therapeutic potential of xanomeline while ameliorating side effects seen in earlier studies. The activity at M1 and M4 receptors indirectly affects dopamine neurotransmission in brain regions involved in mediating symptoms of serious mental illness, such as psychosis in Alzheimer’s disease, as well as the positive, negative, and cognitive symptoms of schizophrenia.
KarXT is currently being evaluated in multiple Phase III clinical trials for psychosis in Alzheimer's disease. The company is currently conducting ADEPT-1 trials with topline data anticipated in 2025 and plans to initiate ADEPT-2 and ADEPT-3 in 2023. Additionally, the drug candidate is also being investigated for the treatment of schizophrenia.
ITI-1284: Intra-Cellular Therapies
ITI-1284, developed by Intra-Cellular Therapeutics, is a deuterated form of lumateperone where carbon-deuterium bonds strategically replace carbon-hydrogen bonds. ITI-1284 has a high affinity for serotonin 5-HT2A receptors and moderate affinity for dopamine D2 and D1 receptors and the serotonin transporter. ITI-1284 is formulated as an oral solid dosage form that dissolves almost instantly when placed under the tongue, allowing for ease of use in the elderly, and may be particularly beneficial for patients who have difficulty swallowing conventional tablets. Currently the drug candidate is being investigated in a Phase II clinical trial to treat psychosis in Alzheimer’s disease.
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Psychosis in Parkinson’s disease and Alzheimer’s disease Drug Class Insights
Pimavanserin is classified as an atypical antipsychotic, specifically known for its unique mechanism of action. Unlike traditional antipsychotics, pimavanserin does not exhibit dopaminergic activity, making it distinctive in its class. It primarily functions as an antagonist and inverse agonist at serotonin receptors, particularly the 5-HT2A and 5-HT2C receptors. This mechanism allows it to manage psychotic symptoms without causing the extrapyramidal side effects commonly associated with other antipsychotic medications.
Atypical antipsychotics primarily function by transiently occupying dopamine D2 receptors and rapidly dissociating from them, allowing normal dopamine neurotransmission to resume. This mechanism reduces the risk of extrapyramidal symptoms associated with typical antipsychotics. Additionally, they antagonize serotonin receptors, particularly 5-HT2A, which helps balance dopamine and serotonin levels, effectively addressing both positive and negative symptoms of schizophrenia and mood disorders while minimizing side effects.
Continued in report…
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Outlook
Psychosis is also common in most people with Alzheimer’s disease throughout their illness. According to studies, over half of the people with Alzheimer’s disease will experience psychotic symptoms during their illness
Antipsychotic medications are the gold-standard treatment for psychotic episodes and disorders, and the choice, dosing, and administration of the medication largely depend on the scenario. Along with medications, family and caregivers also play an important role in managing psychosis, including providing a safe and therapeutic environment. Along with medications, cognitive behavioral therapy is integral in treating patients with psychotic symptoms and is usually recommended as the initial treatment regime.
Although there are no clear guidelines, pimavanserin, 34 mg/day, is the starting point for pharmacotherapy as it is the only FDA-approved drug for Parkinson’s disease Psychosis and is not associated with any serious adverse effects. If ineffective or unavailable, either clozapine (up to 50 mg/day) or quetiapine (slowly up to 150 mg/day) should be considered. Although quetiapine is usually a safe medication, it may not effectively reduce psychotic symptoms in some patients. Similarly, clozapine is associated with the risk of agranulocytosis and may require frequent blood monitoring. There is an urgent need for effective targeted therapy for psychosis in Alzheimer’s disease and psychosis in Parkinson’s disease as current treatment usefulness is limited by their many side effects, such as hallucinations, a feeling of confusion, and nausea. In the absence of approved medications, the development of precise risk and implementation profiles for existing antipsychotics is an essential and immediate goal.
Several drugs are being investigated to cater to the unmet needs of patients with Parkinson’s disease and Alzheimer’s disease psychosis. The late-stage candidates under development include Karuna Therapeutics’ KarXT (xanomeline-trospium), Sunovion Pharmaceuticals’ Ulotaront (SEP-363856), and FANAPT (iloperidone).
- The total Psychosis in Parkinson’s disease and Alzheimer’s disease market size in the 7MM was approximately USD 1,290 million in 2023 and is projected to increase during the forecast period (2024–2034).
- The Psychosis in Parkinson’s disease and Alzheimer’s disease market size in the US was approximately USD 868 million in 2023, which is anticipated to increase due to the increasing awareness of the disease and advancements in treatment options.
- The total Psychosis in Parkinson’s disease and Alzheimer’s disease market size of EU4 and the UK was calculated to be approximately USD 219 in 2023, which was nearly 17% of the total Psychosis in Parkinson’s disease and Alzheimer’s disease market revenue for the 7MM.
- According to DelveInsight’s estimates, among EU4 and the UK, Germany accounted for the highest Psychosis in Parkinson’s disease and Alzheimer’s disease market with approximately USD 71 million in 2023, followed by UK with approximately USD 23 million in the respective year, and the Italy with USD 39 million in 2023.
- According to DelveInsight’s analysis, in the US, among the currently used therapies, the majority of the Psychosis in Parkinson’s disease and Alzheimer’s disease market share was of NUPLAZID, with a revenue of approximately USD 562 million, in 2023.
Psychosis in Parkinson’s disease and Alzheimer’s disease Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the Psychosis in Parkinson’s disease and Alzheimer’s disease market during 2020–2034. For example, Ulotaront (SEP-363856) is expected to enter the US market by 2027 and is projected to have a fast uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Download Psychosis in Parkinson’s disease and Alzheimer’s disease Infographics
Psychosis in Parkinson’s disease and Alzheimer’s disease Pipeline Development Activities
The Psychosis in Parkinson’s disease and Alzheimer’s disease market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The Psychosis in Parkinson’s disease and Alzheimer’s disease market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for psychosis in Parkinson’s disease and Alzheimer’s disease.
KOL Views
To keep up with current Psychosis in Parkinson’s disease and Alzheimer’s disease market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on psychosis in Parkinson’s disease and Alzheimer’s disease evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. University of Texas Health Science Center, Harvard Medical School, University of Cologne, Università di Palermo, King’s College, Fukujuji Hospital, and others were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or psychosis in Parkinson’s disease and Alzheimer’s disease market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Psychosis in Parkinson’s disease and Alzheimer’s disease market and the unmet needs.
Physician’s View
According to physician views on the treatment of psychosis in Alzheimer's disease and Parkinson's disease are managed using atypical antipsychotics such as risperidone, olanzapine, and quetiapine. These medications can help alleviate hallucinations and delusions but may carry risks, including increased mortality in elderly patients with dementia. While psychosis in Parkinson’s disease is a challenging condition; however, it is manageable. Understanding and being on the lookout for new symptoms is often beneficial. The withdrawal from access to readily available treatment interventions is unacceptable and impacts Parkinson’s disease prognosis.
According to a KOL in the US, the recommended first line of therapy for the treatment of psychosis is antipsychotic medicines, also known as neuroleptics. Clozapine and pimavanserin have the best evidence of effectiveness in Parkinson’s disease psychosis for individuals without significant cognitive impairment. Most patients with psychosis who recover must take medication for at least a year. Although some patients may need medication long term to prevent symptoms from reoccurring?
In another KOL in Japan, psychosis is a distressing symptom in Alzheimer’s patients emerging as a neurodegenerative disease before dementia. Nearly 30% of the people living with Alzheimer’s disease in Japan are diagnosed with psychosis. Delusion and hallucinations are among the common neuropsychiatric symptoms. Compared to those without delusions, Alzheimer’s disease patients with delusions had a considerably longer illness duration.”
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Access and Reimbursement
NUPLAZID
In the United States, the Medicare Part D program provides a voluntary outpatient drug benefit to Medicare beneficiaries for certain products. NUPLAZID is available for coverage under Medicare Part D, but the individual Part D plans offer coverage subject to various factors. Medicare Part A and Part B Original Medicare benefits do not provide prescription drug coverage. However, if one gets Part A and Part B benefits through Original Medicare, one can enroll in a stand-alone prescription drug plan (PARKINSON’S DISEASE PSYCHOSIS). If an individual chooses to enroll in a Medicare Advantage plan, the person might have at least the same coverage as Original Medicare, but many include additional benefits, such as prescription drug coverage. Part D plans generally have a monthly premium, and the person may be responsible for deductibles, copayments, and/ or coinsurance. Additionally, a Medicare Part D coverage gap discount program, in which manufacturers agree to offer 70% (commenced from January 1, 2019) point-of-sale discounts to negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for a manufacturer’s outpatient drugs to be covered under Medicare Part D is applicable for NUPLAZID.
Further details will be provided in the report.
The Psychosis in Parkinson’s disease and Alzheimer’s disease market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Psychosis in Parkinson’s disease and Alzheimer’s disease Market Report
- The Psychosis in Parkinson’s disease and Alzheimer’s disease market report covers a segment of key events, an executive summary, and a descriptive overview of psychosis in Parkinson’s disease and Alzheimer’s disease, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the psychosis in Parkinson’s disease and Alzheimer’s disease market, historical and forecasted market size, and market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Psychosis in Parkinson’s disease and Alzheimer’s disease market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM psychosis in the Parkinson’s disease and Alzheimer’s disease market.
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Report insights
- Psychosis in Parkinson’s disease and Alzheimer’s disease Patient Population
- Psychosis in Parkinson’s disease and Alzheimer’s disease Therapeutic Approaches
- Psychosis in Parkinson’s disease and Alzheimer’s disease Pipeline Analysis
- Psychosis in Parkinson’s disease and Alzheimer’s disease Market Size
- Psychosis in Parkinson’s disease and Alzheimer’s disease Market Trends
- Existing and Future Psychosis in Parkinson’s disease and Alzheimer’s disease Market Opportunity
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Report key strengths
- 11 years Forecast
- The 7MM Coverage
- Psychosis in Parkinson’s disease and Alzheimer’s disease Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Psychosis in Parkinson’s disease and Alzheimer’s disease Drugs Uptake
- Key Psychosis in Parkinson’s disease and Alzheimer’s disease Market Forecast Assumptions
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Report Assessment
- Current Psychosis in Parkinson’s disease and Alzheimer’s disease Treatment Practices
- Psychosis in Parkinson’s disease and Alzheimer’s disease Unmet Needs
- Psychosis in Parkinson’s disease and Alzheimer’s disease Pipeline Product Profiles
- Psychosis in Parkinson’s disease and Alzheimer’s disease Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Psychosis in Parkinson’s disease and Alzheimer’s disease Market Insights
- What was the total Psychosis in Parkinson’s disease and Alzheimer’s disease market size, the market size of psychosis in Parkinson’s disease and Alzheimer’s disease by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will Ulotaront (SEP-363856) affect the treatment paradigm of psychosis in Parkinson’s disease and Alzheimer’s disease?
- How will KarXT (xanomeline-trospium) compete with other upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the Psychosis in Parkinson’s disease and Alzheimer’s disease market dynamics and subsequent analysis of the associated trends?
Psychosis in Parkinson’s disease and Alzheimer’s disease Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of psychosis in Parkinson’s disease and Alzheimer’s disease? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to psychosis in Parkinson’s disease and Alzheimer’s disease?
- What is the historical and forecasted psychosis in Parkinson’s disease and Alzheimer’s disease patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent psychosis in Parkinson’s disease and Alzheimer’s disease population during the forecast period (2024–2034)?
- What factors are contributing to the growth of psychosis in Parkinson’s disease and Alzheimer’s disease cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of psychosis in Parkinson’s disease and Alzheimer’s disease? What are the current clinical and treatment guidelines for treating psychosis in Parkinson’s disease and Alzheimer’s disease?
- How many companies are developing therapies for the treatment of psychosis in Parkinson’s disease and Alzheimer’s disease?
- How many emerging therapies are in the mid-stage and late stage of development for treating psychosis in Parkinson’s disease and Alzheimer’s disease?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted psychosis in Parkinson’s disease and Alzheimer’s disease market?
Reasons to Buy
- The Psychosis in Parkinson’s disease and Alzheimer’s disease market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the psychosis in Parkinson’s disease and Alzheimer’s disease market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Psychosis in Parkinson’s disease and Alzheimer’s disease market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying upcoming solid players in the Psychosis in Parkinson’s disease and Alzheimer’s disease market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for psychosis in Parkinson’s disease and Alzheimer’s disease barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Psychosis in Parkinson’s disease and Alzheimer’s disease market so that the upcoming players can strengthen their development and launch strategy.

.jpg)