What Lies Ahead For Parkinson’s Disease Psychosis Treatment Market?
May 26, 2023
The term “psychosis” defines a group of mental symptoms that occur when there has been a partial loss of contact with reality. A person’s thoughts and perceptions are distorted during a psychotic episode, and they could find it difficult to distinguish between tangible and intangible. Other signs include nonsensical or incoherent speech, as well as inappropriate behavior. The diagnosis of psychotic disorders is mostly clinical, i.e., based on the patient’s history, observed behavior, subjective reports, and mental status examination results.
Psychosis in Parkinson’s disease may develop due to both exogenous or endogenous factors and are characterized by hallucinations and delusions. Hallucinations may be vague or well-formed, complex or simple, and usually visual.
There are several risk factors associated with psychosis in Parkinson’s. Some of these include age, illness duration, depression, cognitive impairment, poor vision as well as long-term use of certain anti-parkinsonian medications. The pathogenesis of psychosis is related to the dysfunction of various neurotransmitter systems, including dopamine, acetylcholine, and serotonin (5-hydroxytryptamine, 5-HT), and identifying these dysfunctions is essential for targeted therapies. Dopamine agonists (for example, amphetamine) that are used to relieve Parkinson’s symptoms are also known to produce psychosis, and paradoxically the commonly used antipsychotic agents are blockers of dopamine receptors.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA grants approval; Sanaria receives fast-track designation; Sanofi and Regeneron’s next blockbu...
- Zenas BioPharma’s Obexelimab Delivers Positive Phase 3 Results in IgG4-RD; Sanofi Secures US Prio...
- Novartis Secures FDA Approval for ITVISMA; Kelun-Biotech’s Phase III Study Shows Sac-TMT + Keytru...
- IMFINZI’s AEGEAN Phase III Trial Data for Treating Resectable NSCLC; Ipsen and Day One’s Exclusiv...
- Novo Nordisk’s Concizumab for Hemophilia; AbbVie Ends its Alliance with Alector; ADC Therapeutics...
Although the precise cause of Parkinson’s disease psychosis is unknown, research shows that the CCK-45C-T variant in the cholecystokinin gene is most frequently linked to the condition. Individuals with Parkinson’s disease psychosis exhibit changes in the brain’s visual processing regions as well as disruptions in the cholinergic brain regions. Dysregulation of the sleep-wake cycle, brainstem dysfunction, poor visual processing, and frontal cognitive dysfunction all play a role in the emergence of Parkinson’s disease psychosis.
The primary aim of the management of psychosis in Parkinson’s disease is to reduce the frequency and severity of psychotic symptoms with minimal worsening of motor symptoms. Non-pharmacological approaches such as reality orientation, cognitive stimulation therapy (CST), reminiscence therapy, and psychodynamic therapy are preferred as an initial approach before or at least concurrent with medication. So far, the only US FDA-approved medication for psychosis in Parkinson’s disease treatment is Acadia’s NUPLAZID (pimavanserin), an atypical antipsychotic based on the principle of selective 5-HT2A inverse agonist with no dopaminergic, histaminergic, or muscarinic binding. Atypical antipsychotics are often considered initial therapy, reducing the risk of further deterioration in patients. Apart from these, 5-HT3 receptor antagonists, acetylcholinesterase inhibitors, or other antidepressants are also used.
Epidemiology insights and projection of Parkinson’s disease psychosis
According to the Parkinson’s Disease Foundation, more than 10 million people worldwide are affected by Parkinson’s disease. Of this, 1 million people in the United States suffer from Parkinson’s disease, and roughly 40% suffer from Parkinson’s disease psychosis. Patients with Parkinson’s who develop psychosis have a significantly poor quality of life and add significantly to the burden of the disease. Parkinson’s disease psychosis is also linked with nursing home placement and more stress and responsibility for caregivers.
- Older people are more likely to develop psychotic symptoms than younger people, and Parkinson’s is a progressive disease. It is expected that older patients will also have more advanced Parkinson’s disease and be more likely to develop psychosis.
- Due to Parkinson’s disease psychosis’ high prevalence, timely diagnosis and treatment in the long-term care (LTC) setting is crucial. In reality, two parallel trends—the aging of the population and improved healthcare quality—mean that there will likely be a sharp increase in the number of Parkinson’s disease patients in LTC in the future. The ambiguity in the severity of Parkinson’s disease psychosis will amplify the uncertainty.
- The diagnosed prevalence of Parkinson’s disease psychosis is expected to increase in the coming years. As per DelveInsight’s latest “Psychosis in Parkinson’s and Alzheimer’s Disease Epidemiology Forecast Report,” PDP cases are expected to increase at a CAGR of 2.6%. More than 50% of these cases are observed to be in people ≥70 years of age
- According to the data analyzed, among the 7MM countries, approximately 52% of PDP cases were observed to be in the United States, followed by EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan in 2022.
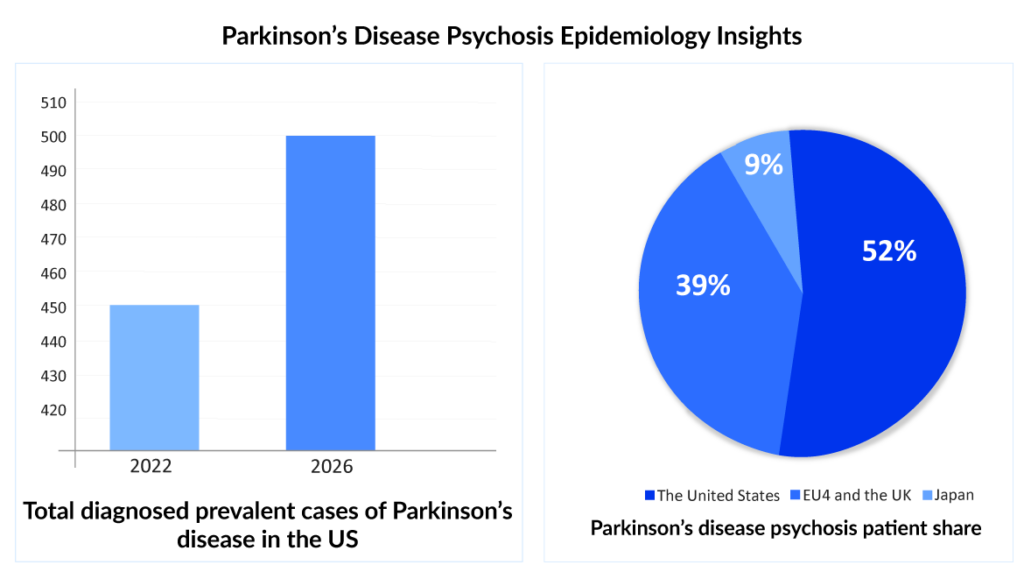
Since some patients are often unaware of their symptoms, while others choose to downplay them and attribute them to an underlying condition or medicine, epidemiological data may also be under-reporting. To accurately establish the worldwide and regional prevalence of psychosis with in-depth patient characteristics and demography analysis, large cohort, population-wide studies are needed.
Market insights and projections of Parkinson’s disease psychosis
The most appropriate treatment for psychotic episodes is antipsychotic drugs, and the selection, administration, and dosing of the drugs depend on the situation. Cognitive behavioral therapy is frequently recommended as the first course of treatment, along with drugs for individuals with psychotic symptoms. Family and carer support is essential in managing psychosis as they create a secure therapeutic environment.
Early identification and treatment of Parkinson’s disease psychosis patients are crucial. Managing Parkinson’s disease psychosis’ medication involves Parkinson’s disease psychosis dopaminergic drug optimization and antipsychotic medication selection. Dopaminergic neurons are found in the substantia nigra, a critical brain region that produces the chemical dopamine. Dopamine is necessary for movement and coordination but does not readily cross the blood-brain barrier. Dopaminergic drugs used to treat the motor impairment brought on by Parkinson’s disease may make psychosis worse. Contrarily, commonly prescribed antipsychotic drugs, which inhibit dopamine receptors, can exacerbate motor symptoms in Parkinson’s disease psychosis patients.
Prior to 2016, the primary treatment of psychosis in Parkinson’s disease was the atypical antipsychotic drugs, quetiapine, and clozapine. In 2016, the US FDA approved NUPLAZID (pimavanserin), a selective 5-HT2A inverse agonist without dopamine receptor-blocking characteristics. The approval gave ACADIA Pharma a first-mover advantage in the Parkinson’s disease psychosis treatment market. A black box warning about increased mortality in older patients with dementia-related psychosis has been given to the drug as it has been reported that these drugs dramatically increase Parkinsonian motor impairment. No specific research has connected pimavanserin to this risk, and the US FDA sees the risk as a class effect.
Acadia Pharmaceuticals is following key strategies for the commercialization and broader reach of NUPLAZID, with sales specialists focused on promoting the drug to neurologists, psychiatrists, and long-term care physicians. The company is leading numerous clinical programs focused on treating neurological and psychiatric diseases that have been neglected and represent significant unmet medical needs. The company is also immersed in licensing and purchasing additional programs via smart business development, like the recent deal with Stoke.
Comparative clinical trials assessing the effectiveness, side effects, and costs of quetiapine, clozapine, and pimavanserin are essential to the success of NUPLAZID. This knowledge will have a big impact on clinical practice and current Parkinson’s disease psychosis therapy recommendations
As per DelveInsight’s latest “Psychosis in Parkinson’s and Alzheimer’s Disease Market Report,” NUPLAZID can become a blockbuster drug by 2027, generating a revenue of more than USD 1 billion. The most important reasons will be a first-mover advantage, a monopoly, and additional regulatory approvals.
Care for patients with Parkinson’s disease psychosis is more expensive than for those with Parkinson’s disease without psychosis. Patients with Parkinson’s disease psychosis used more resources and had higher all-cause expenses. The expenses associated with long-term care (about 116% higher), nursing homes (about 219% higher), and inpatient care (about 68% higher) showed the largest yearly cost differences between Parkinson’s disease psychosis and Parkinson’s disease without psychosis.
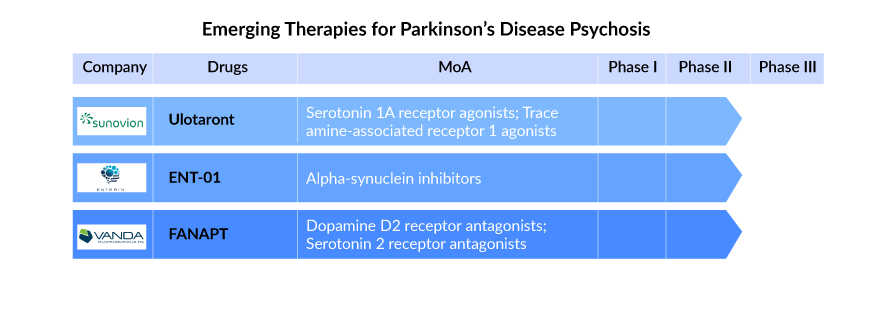
Several drugs are being examined to cater to the unmet needs of patients with Parkinson’s disease psychosis. The late-stage candidates under development include Sunovion Pharmaceuticals’ Ulotaront (SEP-363856) and FANAPT (iloperidone) by Vanda Pharmaceuticals. Among these drugs, as per the analysis by DelveInsight’s analysts, FANAPT has the potential to become a blockbuster for Parkinson’s disease psychosis treatment by generating a revenue of more than a billion after a successful launch in the market. FANAPT is expected to enter the US Parkinson’s disease psychosis treatment market by 2027.
According to DelveInsight’s analysts, the market size of Psychosis in Parkinson’s disease was observed to be approximately USD 500 million in the 7MM for 2022. This is expected to increase in the coming years owing to the successful launch of emerging therapies, the rise in the number of cases, and increasing awareness of the disease. Also, significant progress in genomics, neuroimaging, neurobiological and epidemiological frameworks will lead to novel drug discovery and repurposing approaches
Numerous aspects need to be considered when treating Parkinson’s disease psychosis, and each patient should receive personalized care
- Consider the underlying medical conditions causing or exacerbating the problem (such as an infection, an electrolyte imbalance, or a subdural hematoma). Since Parkinson’s disease psychosis is not frequently accompanied by an altered degree of consciousness, its presence should provoke a search for medical causes for delirium or the possibility of an alternate diagnosis, such as dementia with Lewy bodies (DLB).
- Review of medications and efforts to prevent taking any unnecessary centrally-acting drugs. Drugs for a specific condition should be chosen whenever possible because they are less likely to worsen the mental status. For the treatment of rapid eye movement (REM) sleep behavior disorder (RBD) in Parkinson’s disease psychosis, for instance, melatonin should be preferred to clonazepam.
- Modifications to Parkinson’s disease medicines as motor function permits. If treatment was modified before the onset or exacerbation of psychosis, causality should be considered, and adjustments should be made as necessary. Otherwise, specialists advise stopping adjunctive Parkinson’s disease medications. It is good to weigh the relative potential benefits toward motor function and risks for worsening psychosis to decide how to stop taking anticholinergics, monoamine oxidase-B inhibitors, amantadine, dopamine agonists, and COMT inhibitors. Carbidopa–levodopa dosage adjustments should be saved for last.
- Counseling for patients and carers on the diagnosis and suggestions for behavioral and environmental changes. Patients with visual hallucination (VH) may benefit from avoiding low-light conditions, correcting any vision issues, and/or being subjected to reality testing that includes the use of cognitive, interactive (carers’ involvement), and/or visual tactics.
While standardized diagnostic procedures and international consensus standards for psychosis exist, clinical practice cannot employ the present assessment methods for psychosis as they are too complex. There is a need for innovative, well-tolerated medicines that produce a persistent reduction in symptoms, are efficacious and have improved safety and tolerability profiles. With a continuous focus on genetics and precision medicine, there is hope for new targeted treatments. The present lack of curative therapies and the significant side effects of the existing therapies creates potential for pharmaceutical companies to develop optimal Parkinson’s disease psychosis treatments that will delay and prevent disability while keeping psychosis in check.
FAQs
Parkinson’s disease psychosis is a prevalent occurrence in Parkinson’s disease patients that is associated with significant morbidity and mortality. It is caused by anomalies in dopamine, serotonin, and glutamate neurotransmission induced by Lewy body deposition in the brain.
Due to the complexity of the etiologies and physiological hypotheses, many diagnostic procedures, including neuroimaging, neurophysiological, genotypic, and serologic examinations, are used to diagnose psychosis in Alzheimer’s disease and Parkinson’s disease patients.
The treatment’s major goal is to lessen the frequency and severity of psychotic symptoms while causing minimal worsening of motor symptoms in Parkinson’s disease psychosis and Alzheimer’s disease psychosis patients. Clozapine, quetiapine, olanzapine, risperidone, and aripiprazole are examples of atypical antipsychotics. Clozapine has been extensively examined and is regarded as the “gold standard” for Parkinson’s disease psychosis treatment.
NUPLAZID (pimavanserin), an atypical antipsychotic, is the sole FDA-approved treatment for Parkinson’s disease psychosis and is also being tested for Alzheimer’s disease psychosis. It functions on the basis of a selective 5-HT2A inverse agonist with nodopaminergic, histaminergic, or muscarinic binding.

Downloads
Article in PDF
Recent Articles
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
- What is the Link Between Alzheimer’s and Menopause?
- How are Technological Trends and Innovations Reshaping the Dementia Care?
- Chimerix Submits Dordaviprone NDA for Accelerated Approval in Recurrent H3 K27M-Mutant Diffuse Gl...
- Decoding Parkinson’s Diagnosis with Gene Therapies and Prevention Insights




