Sanfilippo Syndrome Market Summary
- According to DelveInsight, the Sanfilippo Syndrome market in 7MM is expected to witness a major change in the study period 2019-2032.
- Sanfilippo Syndrome Companies are Ultragenyx, JCR Pharmaceuticals, BioMarin Pharmaceutical, Denali Therapeutics, Alexion Pharmaceuticals, and GC Biopharma and others.
Sanfilippo Syndrome Market Insights and Trends
- Sanfilippo Syndrome Market Growth is driven by increased research funding, improved diagnostics, a rising demand for advanced therapies like gene and enzyme replacement, and a growing number of clinical trials
- Emerging therapies: Therapies like SLS-005 (trehalose), Lysogene (LYS-SAF302), and Tralesinidase alfa (BMN 250) are expected to launch and boost the market.
Factors Affecting Sanfilippo Syndrome Market Growth
Increasing Awareness & Improved Genetic Diagnosis
Growing use of newborn screening, next-generation sequencing (NGS), and broader genetic testing panels leads to earlier and more accurate identification of Sanfilippo Syndrome (MPS III), expanding the diagnosed patient pool.
Strong Unmet Need in a Fatal Neurodegenerative Disorder
With no approved disease-modifying therapy currently available, there is high clinical and patient demand for effective treatments—driving R&D and market potential.
Advancements in Gene Therapy & Novel Therapeutics
Ongoing development of AAV-based gene therapies, enzyme replacement therapies (ERT), intrathecal approaches, substrate reduction therapies (SRT), and anti-inflammatory agents significantly boosts pipeline growth and investor interest.
Supportive Regulatory Environment
Orphan drug designations, fast-track approvals, rare pediatric disease vouchers (PRVs), and regulatory incentives encourage companies to develop treatments for MPS III.
Increasing Patient Advocacy & Global Registries
Organizations like the Cure Sanfilippo Foundation, Team Sanfilippo, and others improve trial recruitment, awareness, fundraising, and global natural history studies, strengthening the ecosystem.
DelveInsight's "Sanfilippo Syndrome Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of the Sanfilippo Syndrome, historical and forecasted epidemiology as well as the Sanfilippo Syndrome market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
The Sanfilippo Syndrome market report provides current treatment practices, emerging drugs, Sanfilippo Syndrome market share of the individual therapies, current and forecasted Sanfilippo Syndrome market Size from 2019 to 2032 segmented by seven major markets. The Report also covers current Sanfilippo Syndrome treatment practice/algorithm, market drivers, market barriers and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the Sanfilippo Syndrome market.
|
Study Period |
2019 to 2032 |
|
Forecast Period |
2023-2032 |
|
Geographies Covered |
|
|
Sanfilippo Syndrome Market |
|
|
Sanfilippo Syndrome Market Size | |
|
Sanfilippo Syndrome Companies |
Ultragenyx, JCR Pharmaceuticals, BioMarin Pharmaceutical, Denali Therapeutics, Alexion Pharmaceuticals, and GC Biopharma and others. |
|
Sanfilippo SyndromeEpidemiology Segmentation |
|
Sanfilippo Syndrome Treatment Market
Mucopolysaccaridosis type III (MPS III), also known as Sanfilippo syndrome, is a rare genetic condition that causes fatal brain damage. It is a type of childhood dementia. MPS III is caused by a lack of an enzyme that normally breaks down and recycles a large, complex sugar molecule called ‘heparan sulphate’. This heparan sulphate accumulates and causes damage to the cells of the central nervous system, including the brain. Sanfilippo belongs to a group of disorders known as the “mucopolysaccharidoses” (MPS), which are part of a larger group of disorders known as “lysosomal storage disorders”.
Sanfilippo Syndrome Diagnosis
This segment of the Sanfilippo Syndrome market report covers the detailed diagnostic methods or tests for Sanfilippo Syndrome. Diagnosis of this syndrome often involves a combination of clinical evaluation, genetic testing, and measurement of elevated levels of heparan sulfate in the urine. Genetic testing can determine the specific subtype of Sanfilippo syndrome. The diagnosis may be confirmed by enzyme assay of skin fibroblasts and white blood cells.
Sanfilippo Syndrome Treatment
It covers the details of conventional and current medical therapies available in the Sanfilippo Syndrome market for the treatment of the condition. It also provides Sanfilippo Syndrome treatment algorithms and guidelines in the United States, Europe, and Japan. Currently, there is no cure for Sanfilippo syndrome. Treatment primarily focuses on managing symptoms and providing supportive care. Therapies such as speech and occupational therapy, behavioral interventions, and medications to manage certain symptoms may be helpful. Various gene therapies and enzyme replacement therapies are currently under development for Sanfilippo syndrome.
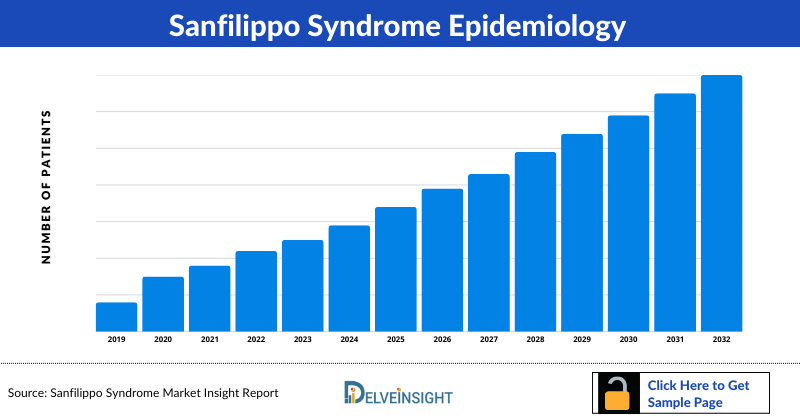
Sanfilippo Syndrome Epidemiology
The Sanfilippo Syndrome epidemiology section provides insights about the historical and current Sanfilippo Syndrome patient pool and forecasted trends for individual seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the Sanfilippo Syndrome market report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
The disease epidemiology covered in the Sanfilippo Syndrome market report provides historical as well as forecasted Sanfilippo Syndrome epidemiology scenario in the 7MM covering the United States, EU5 countries (Germany, Spain, Italy, France, and the United Kingdom), and Japan from 2019 to 2032.
- The findings from National Organization for Rare Disorders also quoted that MPS IIIA is the most common subtype affecting around 1 in 100,000 births, closely followed by type B at 1 in 200,000.
- According to Celik et al. (2021), in France, the prevalence of MPS III was found to be 0.73 per 100,000 live births.
- As per research conducted by Khan et al. (2017), in Japan, the birth prevalence was found to be 1 out of 385,000 for all MPS III types (1 out of 1,000,000 for MPS IIIA, 1 out of 834,000 for MPS IIIB, 1 out of 2.5 million for MPS IIIC).
- According to a retrospective study by Puckett et al. (2021), in the United States using the National MPS Society database records, the prevalence of MPS III (Sanfilippo syndrome) was found to be 0.71 per 1,000,000. Subtypes MPS IIIA, MPS IIIB, MPS IIIC, and MPS IIID had prevalence of 0.52, 0.14, 0.04, and 0 per 1,000,000.
Country Wise- Sanfilippo Syndrome Epidemiology
The epidemiology segment also provides the Sanfilippo Syndrome epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan.
Sanfilippo Syndrome Drug Analysis
The drug chapter segment of the Sanfilippo Syndrome drugs market report encloses the detailed analysis of marketed Sanfilippo Syndrome drugs and late-stage (Phase-III and Phase-II) Sanfilippo Syndrome pipeline drugs. It also helps to understand the Sanfilippo Syndrome clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Sanfilippo Syndrome Drugs
The Sanfilippo Syndrome market report provides the details of the marketed products/off-label treatments available for Sanfilippo Syndrome treatment.
Emerging Sanfilippo Syndrome Drugs
The Sanfilippo Syndrome market report provides the details of the emerging therapies under the late and mid-stage of development for Sanfilippo Syndrome treatment.
ABO-102 (UX111): Abeona Therapeutics
ABO-102 (UX111), being developed by Abeona Therapeutics, is a novel gene therapy in development for Sanfilippo syndrome type A (MPS IIIA), a rare lysosomal storage disease with no approved treatment that primarily affects the central nervous system (CNS). ABO-102 is dosed in a one-time intravenous infusion using a self-complementary AAV9 vector to deliver a functional copy of the SGSH gene to cells of the CNS and peripheral organs. The therapy is designed to address the underlying SGSH enzyme deficiency responsible for abnormal accumulation of glycosaminoglycans in the brain and throughout the body that results in progressive cell damage and neurodevelopmental and physical decline. The ABO-102 program has received Regenerative Medicine Advanced Therapy, Fast Track, Rare Pediatric Disease, and Orphan Drug designations in the US, and PRIME and Orphan medicinal product designations in the EU. In May 2022, Ultragenyx acquired global rights to AAV Gene Therapy ABO-102 for Sanfilippo Syndrome Type A (MPS IIIA) from Abeona Therapeutics. Currently, the drug is being evaluated in Phase II/III trial (NCT02716246) for the treatment of MPS IIIA. The company is also conducting a Phase III (NCT04360265) long-term follow-up study of ABO-102 in participants with MPS IIIA.
AX 250 (tralesinidase alfa): BioMarin Pharmaceutical
AX 250 (tralesinidase alfa) , originally developed by BioMarin Pharmaceutical and now the exclusive worldwide license owned by Allievex, is an enzyme replacement therapy. The enzyme is fused to a protein fragment called insulin-like growth factor-2, which is supposed to help the enzyme be taken up by the right cellular compartments. It is delivered through intracerebroventricular (ICV) administration, where the drug is injected into an Ommaya reservoir to bypass the blood-brain barrier and send medication into the cerebrospinal fluid that bathes cells of the central nervous system. The company has completed a Phase I/II (NCT02754076) of AX 250 in patients with MPS IIIB. The drug was found to safely reduce liver size, slow brain shrinkage, and demonstrate the ability to stabilize cognitive function among children with Sanfilippo syndrome type B. The company is currently conducting a treatment extension study of AX 250 for MPS III B.
JR-441: JCR Pharmaceuticals
JR-441, developed by JCR Pharmaceuticals, is a recombinant fusion protein of antibody against the human transferrin receptor and heparan N sulfatase. By crossing the blood-brain barrier, JR-441 is expected to be effective also against CNS symptoms of the disease, thereby addressing a significant unmet need in the treatment of MPS IIIA. Recently, in July 2023, the CTA for a Phase I/II clinical trial submitted in Germany has been approved by the PEI and JCR is finalizing the protocol and other requirements with the aim to start recruiting near the third quarter of 2023. JR-441 has also been granted ODD by the European Commission to treat MPS IIIA.
Sanfilippo Syndrome Market Outlook
The Sanfilippo Syndrome market outlook of the report helps to build a detailed comprehension of the historic, current, and forecasted Sanfilippo Syndrome market trends by analyzing the impact of current Sanfilippo Syndrome therapies on the market, unmet needs, drivers and barriers, and demand for better technology.
This segment gives a thorough detail of Sanfilippo Syndrome market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on the annual cost of therapy, inclusion and exclusion criteria's, Sanfilippo Syndrome mechanism of action, compliance rate, growing need of the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the market and view of the key opinion leaders. The calculated Sanfilippo Syndrome market data are presented with relevant tables and graphs to give a clear view of the Sanfilippo Syndrome market at first sight.
There is ongoing research into potential therapies for Sanfilippo syndrome, including enzyme replacement therapy, Substrate reduction therapy, and gene therapy. Expected Launch of potential Sanfilippo Syndrome therapies may increase the Sanfilippo Syndrome treatment market size in the coming years, assisted by an increase in prevalence of Sanfilippo syndrome. Owing to the positive outcomes of the several products during the developmental stage by key Sanfilippo Syndrome companies such as Abeona Therapeutics, Ultragenyx, Allievex, JCR Pharmaceuticals, and others, the market is expected to witness a significant positive shift in the Sanfilippo Syndrome Market Size.
According to DelveInsight, the Sanfilippo Syndrome market in 7MM is expected to witness a major change in the study period 2019-2032.
Key Findings
This section includes a glimpse of the Sanfilippo Syndrome market in 7MM.
The United States Sanfilippo Syndrome Market Outlook
This section provides the total Sanfilippo Syndrome market size and market size by therapies in the United States.
EU-5 Countries Sanfilippo Syndrome Market Outlook
The total Sanfilippo Syndrome market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom is provided in this section.
Japan Sanfilippo Syndrome Market Outlook
The total Sanfilippo Syndrome market size and market size by therapies in Japan is also mentioned.
Sanfilippo Syndrome Drugs Uptake
This section focuses on the rate of uptake of the potential Sanfilippo Syndrome drugs recently launched in the Sanfilippo Syndrome market or expected to get launched in the market during the study period 2019-2032. The analysis covers Sanfilippo Syndrome market uptake by drugs; patient uptake by therapies; and sales of each drug.
Sanfilippo Syndrome Drugs Uptake helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allow the comparison of the drugs on the basis of Sanfilippo Syndrome market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Sanfilippo Syndrome Clinical Trial Activities
The Sanfilippo Syndrome treatment market report provides insights into different therapeutic candidates in Phase II, and Phase III stage. It also analyses Sanfilippo Syndrome companies involved in developing targeted therapeutics.
Sanfilippo Syndrome Pipeline Development Activities
The Sanfilippo Syndrome drugs market report covers the detailed information of collaborations, acquisition, and merger, licensing, patent details, and other information for emerging Sanfilippo Syndrome therapies.
Reimbursement Scenario in Sanfilippo Syndrome
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we take reimbursement into consideration to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
Latest KOL- Views on Sanfilippo Syndrome
To keep up with current Sanfilippo Syndrome market trends, we take KOLs and SMEs ' opinion working in the Sanfilippo Syndrome domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Sanfilippo Syndrome market trends. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the Sanfilippo Syndrome market and the unmet needs.
Sanfilippo Syndrome Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Sanfilippo Syndrome Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Sanfilippo Syndrome Market Report
- The Sanfilippo Syndrome market report covers the descriptive overview of Sanfilippo Syndrome, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available Sanfilippo Syndrome therapies
- Comprehensive insight has been provided into the Sanfilippo Syndrome epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Sanfilippo Syndrome is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Sanfilippo Syndrome market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The Sanfilippo Syndrome therapeutics market report provides an edge while developing business strategies, by understanding trends shaping and driving the global Sanfilippo Syndrome market
Sanfilippo Syndrome Market Report Highlights
- In the coming years, the Sanfilippo Syndrome market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The Sanfilippo Syndrome companies and academics are working to assess challenges and seek opportunities that could influence Sanfilippo Syndrome R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major Sanfilippo Syndrome companies are involved in developing therapies for Sanfilippo Syndrome. The launch of emerging therapies will significantly impact the Sanfilippo Syndrome market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Sanfilippo Syndrome
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Sanfilippo Syndrome Market Report Insights
- Sanfilippo Syndrome Patient Population
- Therapeutic Approaches
- Sanfilippo Syndrome Pipeline Analysis
- Sanfilippo Syndrome Market Size
- Sanfilippo Syndrome Market Trends
- Sanfilippo Syndrome Market Opportunities
- Impact of upcoming Sanfilippo Syndrome Therapies
Sanfilippo Syndrome Market Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Sanfilippo Syndrome Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Sanfilippo Syndrome Market
- Sanfilippo Syndrome Drugs Uptake
Sanfilippo Syndrome Market Report Assessment
- Current Treatment Practices
- Sanfilippo Syndrome Unmet Needs
- Sanfilippo Syndrome Pipeline Product Profiles
- Sanfilippo Syndrome Market Attractiveness
- Sanfilippo Syndrome Market Drivers
- Sanfilippo Syndrome Market Barriers
Key Questions Answered in the Sanfilippo Syndrome Market Report
Sanfilippo Syndrome Market Insights
- What was the Sanfilippo Syndrome drug class share (%) distribution in 2019 and how it would look like in 2032?
- What would be the Sanfilippo Syndrome market size as well as market size by therapies across the 7MM during the forecast period (2019-2032)?
- What are the key findings pertaining to the market across 7MM and which country will have the largest Sanfilippo Syndrome market size during the forecast period (2019-2032)?
- At what CAGR, the Sanfilippo Syndrome market is expected to grow by 7MM during the forecast period (2019-2032)?
- What would be the Sanfilippo Syndrome market outlook across the 7MM during the forecast period (2019-2032)?
- What would be the Sanfilippo Syndrome market growth till 2032, and what will be the resultant Sanfilippo Syndrome market Size in the year 2032?
- How would the unmet needs affect the Sanfilippo Syndrome market dynamics and subsequent analysis of the associated trends?
Sanfilippo Syndrome Epidemiology Insights
- What are the disease risk, burden, and regional/ethnic differences of the Sanfilippo Syndrome?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What is the historical Sanfilippo Syndrome patient pool in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What would be the forecasted patient pool of Sanfilippo Syndrome in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- Where will be the growth opportunities in the 7MM with respect to the patient population pertaining to Sanfilippo Syndrome?
- Out of all 7MM countries, which country would have the highest prevalent population of Sanfilippo Syndrome during the forecast period (2019-2032)?
- At what CAGR the Sanfilippo Syndrome patient population is expected to grow in 7MM during the forecast period (2019-2032)?
Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current options for the Sanfilippo Syndrome treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of Sanfilippo Syndrome in the USA, Europe, and Japan?
- What are the Sanfilippo Syndrome marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety and efficacy, etc.?
- How many Sanfilippo Syndrome companies are developing therapies for the treatment of Sanfilippo Syndrome?
- How many Sanfilippo Syndrome therapies are in-development by each company for Sanfilippo Syndrome treatment?
- How many are emerging Sanfilippo Syndrome therapies in mid-stage, and late stage of development for Sanfilippo Syndrome treatment?
- What are the key collaborations (Industry - Industry, Industry - Academia), Mergers and acquisitions, licensing activities related to the Sanfilippo Syndrome therapies?
- What are the recent novel therapies, targets, Sanfilippo Syndrome mechanisms of action and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Sanfilippo Syndrome and their status?
- What are the current challenges faced in Sanfilippo Syndrome drug development?
- What are the key designations that have been granted for the emerging Sanfilippo Syndrome therapies?
- What are the global historical and forecasted Sanfilippo Syndrome treatment market?
Reasons to buy the Sanfilippo Syndrome Market Report
- The Sanfilippo Syndrome market report will help in developing business strategies by understanding trends shaping and driving the Sanfilippo Syndrome market
- To understand the future market competition in the Sanfilippo Syndrome market and Insightful review of the key Sanfilippo Syndrome market drivers and barriers
- Organize Sanfilippo Syndrome sales and marketing efforts by identifying the best opportunities for Sanfilippo Syndrome in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom) and Japan
- Identification of strong upcoming Sanfilippo Syndrome companies in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for Sanfilippo Syndrome market
- To understand the future market competition in the Sanfilippo Syndrome market


-pipeline.png&w=256&q=75)

