Severe Hypertriglyceridemia Market Summary
- The Severe Hypertriglyceridemia Market has notable unmet needs, and with the anticipated approval of these new therapies, substantial growth is projected as they gain traction in clinical use.
- The leading Severe Hypertriglyceridemia Companies such as Ionis Pharmaceuticals, Arrowhead Pharmaceuticals, and NorthSea Therapeutics B.V., and others.
Severe Hypertriglyceridemia Market Insights & Epidemiology Forecast
- in the 7MM were around 3.6 million in the 2023, of which the US accounted for the highest number of cases.
- Severe Hypertriglyceridemia Treatment typically includes dietary changes and lipid-lowering drugs like fibrates, omega-3 fish oils, and niacin, though statins.
- The Severe Hypertriglyceridemia Treatment Market in this disease area has been dominated by statins, followed by fibrates, niacin, and omega-3 fatty acids.
- Despite the availability of treatments like statins, fibrates, and omega-3 fatty acids, SHTG continues to significantly impact patients’ quality of life, underscoring a significant unmet need in current therapies.
- VASCEPA (icosapent ethyl) is FDA-approved for SHTG and cardiovascular risk reduction. However, it only modestly lowers triglycerides and carries risks like atrial fibrillation and bleeding.
- Olezarsen holds a first-mover advantage in the Severe Hypertriglyceridemia Market and is expected to become the first blockbuster drug in its class. Its introduction could also benefit plozasiran by enhancing mutual marketing initiatives, potentially influencing physicians to opt for triglyceride-lowering therapies instead of the more frequently prescribed statins.
- Plozasiran is the only siRNA therapy in development for SHTG, offering a promising new treatment option. As it nears market entry, it is set to compete directly with Ionis Pharmaceuticals’ olezarsen.
- To address the unmet needs in Severe Hypertriglyceridemia Companies are different types of molecules, including antisense oligonucleotides, small interfering RNAs, and recombinant proteins.
Request for Unlocking the Sample Page of the "Severe Hypertriglyceridemia Treatment Market"
Key Factors Driving the Severe Hypertriglyceridemia Market Growth
-
Rising Prevalence of Hypertriglyceridemia
Increasing cases of elevated triglyceride levels worldwide, driven by sedentary lifestyles, obesity, diabetes, and poor dietary habits, are expanding the patient pool for treatment.
-
Growing Awareness of Cardiovascular Risks
Heightened awareness of the link between severe hypertriglyceridemia and cardiovascular diseases, including pancreatitis, is driving early diagnosis and management.
-
Advancements in Diagnostic Tools
Improved laboratory testing and lipid profiling enable timely identification of high-risk patients, leading to increased treatment initiation.
-
Introduction of Novel Therapies
The development and approval of new lipid-lowering agents and therapies specifically targeting triglyceride reduction are expanding treatment options and fueling market growth.
-
Strong Research and Development Pipeline
Ongoing clinical trials focusing on innovative therapies for severe hypertriglyceridemia provide optimism for improved outcomes, encouraging investment and adoption.
-
Increasing Geriatric and Obese Population
The global rise in obesity and aging populations contributes to higher prevalence of metabolic disorders, including severe hypertriglyceridemia, thereby increasing market demand.
-
Supportive Regulatory Environment
Favorable regulatory frameworks for novel lipid-lowering drugs are facilitating faster approvals and wider market availability.
-
Growing Focus on Preventive Healthcare
Greater emphasis on early intervention and long-term management of lipid disorders is driving adoption of both pharmacological and lifestyle-based treatment strategies.
DelveInsight's “Severe Hypertriglyceridemia Treatment Market Insights, Epidemiology and Market Forecast– 2034” report delivers an in-depth understanding of the SHTG, historical and forecasted epidemiology as well as the SHTG market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Severe Hypertriglyceridemia Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM SHTG market size from 2020 to 2034. The report also covers current SHTG treatment market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Scope of the Severe Hypertriglyceridemia Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Severe Hypertriglyceridemia Market |
|
|
Severe Hypertriglyceridemias Market Size | |
|
Severe Hypertriglyceridemia Companies |
|
|
Severe Hypertriglyceridemia Epidemiology Segmentation |
|
Severe Hypertriglyceridemia Treatment Market
High triglycerides are the characteristic condition for the disease called hypertriglyceridemia. Severe Hypertriglyceridemia Patients have triglyceride levels more than three times the normal level. It leads to multiple serious conditions, including cardiovascular disease (CVD) and acute pancreatitis. High triglycerides may contribute to the hardening of the arteries or the thickening of the artery walls (arteriosclerosis) — which increases the risk of stroke, heart attack, and heart disease. SHTG does not have noticeable symptoms; however, individuals with extremely high levels of triglycerides (greater than 2,000 mg/dL) can experience recurrent abdominal pain, nausea, vomiting, xanthomas (yellow-colored bumps on the skin), and acute pancreatitis.
Severe Hypertriglyceridemia Diagnosis
Hypertriglyceridemia is diagnosed by doing blood tests to check very low-density lipoprotein (VLDL) and triglyceride levels. Blood tests show a mild to moderate increase in triglycerides (about 200–500 mg/dL). A triglycerides test is usually part of a lipid profile. A triglycerides test is a blood test. During the test, a healthcare professional will take a blood sample from a vein in the arm using a small needle. After the needle is inserted, a small amount of blood will be collected into a test tube or vial. Patients may feel a little sting when the needle goes in or out. This usually takes less than five minutes.
The patient journey is categorized into genetically based disorders (primary disorders) and secondary disorders due to other diseases. Lipoprotein lipase (LPL) deficiency and Apolipoprotein (Apo) C-II deficiency are two well-genetically characterized kinds of SHTG that usually present in infancy as chylomicronemia syndromes causing SHTG at a very early childhood age. Nausea, vomiting, etc affect the patient. The Severe Hypertriglyceridemia patient then is diagnosed by triglyceride test, where doctors are able to find the levels of triglycerides and confirm if the patient has SHTG. There are various clinical practice guidelines for the management of hypertriglyceridemia and the role of prescription omega-3 fatty acids in preventing pancreatitis and CV disease in individuals with high and very high TG levels.
Further details related to country-based variations are provided in the report
Severe Hypertriglyceridemia Treatment
The Severe Hypertriglyceridemia treatment includes dietary restrictions and lipid-lowering drug treatment such as the use of medium-chain triglycerides (MCT), fibrates, omega-3-fatty acids (omega-3-FA), and nicotinic acid. Severe Hypertriglyceridemia Drugs like fenofibrate, Lovaza, VASCEPA, etc. are approved. Severe Hypertriglyceridemia Patients with very high triglyceride levels (i.e., 500 mg per dL or higher) usually require drug therapy in addition to therapeutic lifestyle changes. Fibrates or niacin is a practical first-line choice for these patients.
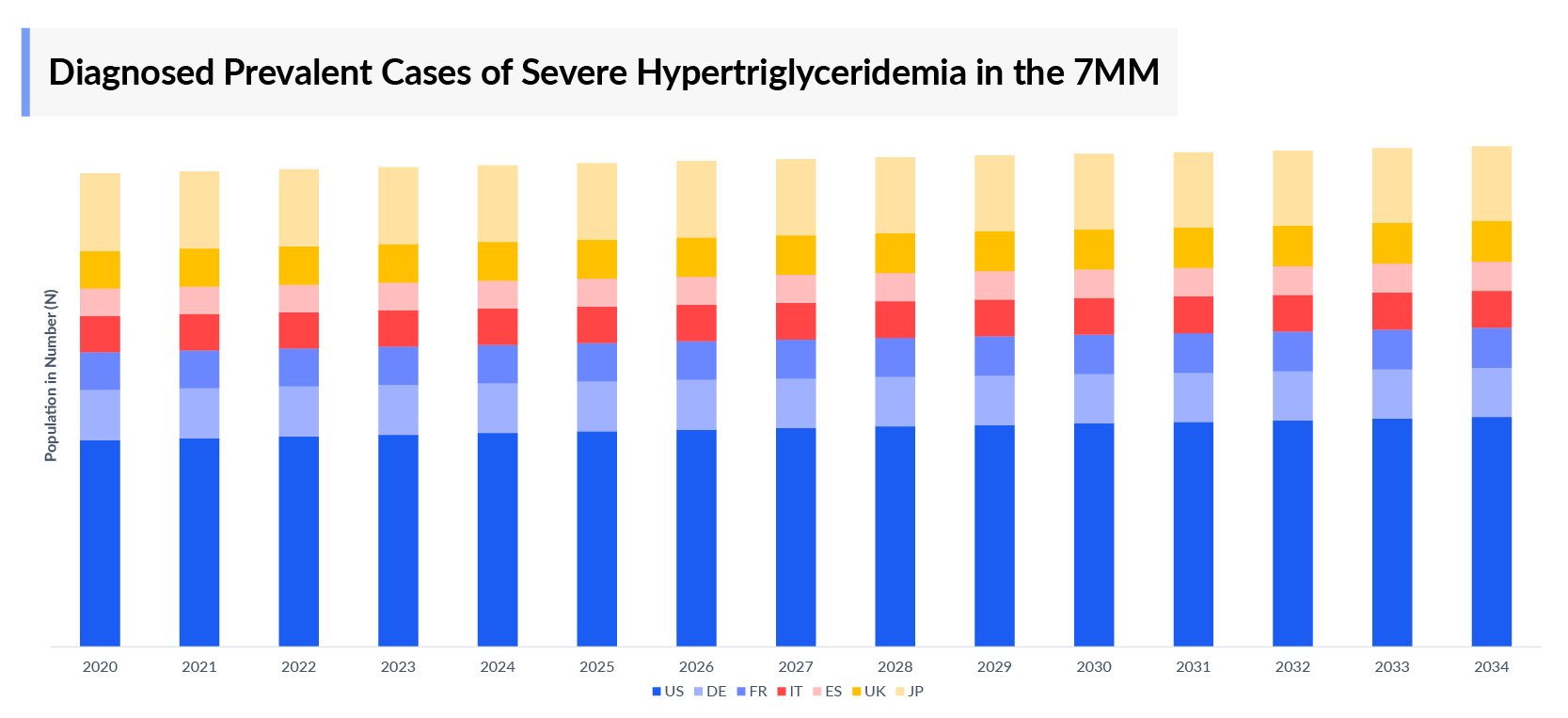 Severe Hypertriglyceridemia Epidemiology
Severe Hypertriglyceridemia Epidemiology
The SHTG epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total Severe Hypertriglyceridemia Prevalence Cases, age-specific diagnosed prevalent cases of SHTG, and gender-specific diagnosed prevalent cases of SHTG in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
Key findings from Severe Hypertriglyceridemia Epidemiological Analysis
- The total number of Severe Hypertriglyceridemia diagnosed prevalent cases in the 7MM was nearly 3.6 million cases in 2023 and is projected to increase during the forecast period.
- In 2023, the Severe Hypertriglyceridemia diagnosed prevalent cases were highest in the US among the 7MM, with 2.7 million cases.
- Among EU4 and the UK, the highest number of cases of SHTG was found in Germany whereas Spain accounted for the lowest cases in 2023.
- A significant portion of Severe Hypertriglyceridemia patients remain undiagnosed. Among those who are diagnosed, approximately 50% do not receive treatment. This highlights a major gap in both diagnosis and management of the condition.
- Among the type-specific cases, the number of secondary SHTG cases was higher than that of primary SHTG.
Severe Hypertriglyceridemia Drugs Analysis
The drug chapter segment of the Severe Hypertriglyceridemia Therapeutics Market Report encloses a detailed analysis of the marketed, late, and mid-stage (Phase III, Phase II) Severe Hypertriglyceridemia pipeline drugs such as ANGPTL3 Inhibitors. The marketed drugs segment encloses only VASCEPA/VAZKEPA. The current emerging candidates are Olezarsen, Plozasiran, Pegozafermin, and SEFA-1024. The drug chapter also helps understand the Severe Hypertriglyceridemia clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Severe Hypertriglyceridemia Marketed Drugs
-
VASCEPA/VAZKEPA: Amarin Corporation
VASCEPA is an ethyl ester of eicosapentaenoic acid (EPA) indicated as an orally administered adjunct to diet to reduce TG levels in adult patients with severe (=500 mg/dL) hypertriglyceridemia. It is the first and only FDA-approved medication for reducing cardiovascular risk beyond cholesterol-lowering therapy in high-risk patients approved for treatment. In July 2012, VASCEPA was approved by the US FDA for the reduction of triglyceride levels in patients with =500 mg/dL. In March 2021, marketing authorization was granted to icosapent ethyl in the European Union to reduce the risk of cardiovascular events in patients at high cardiovascular risk under the brand name VAZKEPA. In the same year, the company launched VAZKEPA in Germany.
In December 2020, Amarin sent a letter to the payer community, including Envolve, the PBM that Health Net, on information and belief, uses to manage its pharmacy benefits, concerning the launch of the Hikma Defendants’ generic version of VASCEPA. In August 2024, PBM informed that it no longer covered VASCEPA as the exclusive icosapent ethyl product for its commercial national formularies and has transitioned VASCEPA to not cover.
Severe Hypertriglyceridemia Emerging Drugs
-
Olezarsen: Ionis Pharmaceuticals
Olezarsen is an RNA-targeted investigational LIgand Conjugated Antisense (LICA) medicine being evaluated for people at risk of disease due to elevated triglyceride levels, including those with FCS and SHTG. Currently, it is being investigated in Phase III for SHTG. As of October 2024, Ionis Pharmaceuticals expects to release Phase III data for olezarsen in 2025 and the company also anticipates its potential US approval for its use in treating SHTG between 2026 and 2027.
-
Plozasiran: Arrowhead Pharmaceuticals
Plozasiran (formerly ARO-APOC3) is designed to reduce the production of the protein ApoC-III through the natural RNA interference (RNAi) mechanism. Currently, the drug is investigated in Phase III. Arrowhead Pharmaceuticals is working on initiating SHASTA-5, a Phase III study in patients with SHTG who are at high risk of acute pancreatitis. According to the August 2024 presentation, Arrowhead Pharmaceuticals is expecting commercialization with growth for plozasiran for SHTG in 2027.
Note: Detailed emerging therapies assessment will be provided in the final report.
Severe Hypertriglyceridemia Drugs Market Insights
The existing Severe Hypertriglyceridemia treatment mainly includes different classes of drugs for treating SHTG. These classes’ are statins, fibrates, omega-3 fatty acids, and niacin. Currently, Statins lead the SHTG market, followed by fibrates, niacin and omega-3 fatty acids. Despite the availability of treatments like statins, fibrates, and omega-3 fatty acids, SHTG continues to significantly impact patients’ quality of life, underscoring a significant unmet need in current therapies.
- ApoC3 Inhibitors
Ionis Pharmaceuticals is developing olezarsen, an antisense therapy targeting ApoC-III to reduce triglycerides by inhibiting its production in the liver. In trials, monthly injections have shown significant triglyceride reductions in patients with both moderate and severe hypertriglyceridemia, positioning olezarsen as a strong contender in the market. With FDA Priority Review and expected approval in December for FCS, olezarsen could secure a first-mover advantage, potentially launching ahead of competitors like plozasiran. Suppose its Phase III results in SHTG are positive. In that case, olezarsen is well-positioned to dominate the market and become the standard of care for both FCS and the broader SHTG population. This gives Ionis a competitive edge in addressing the unmet needs in this therapeutic area.
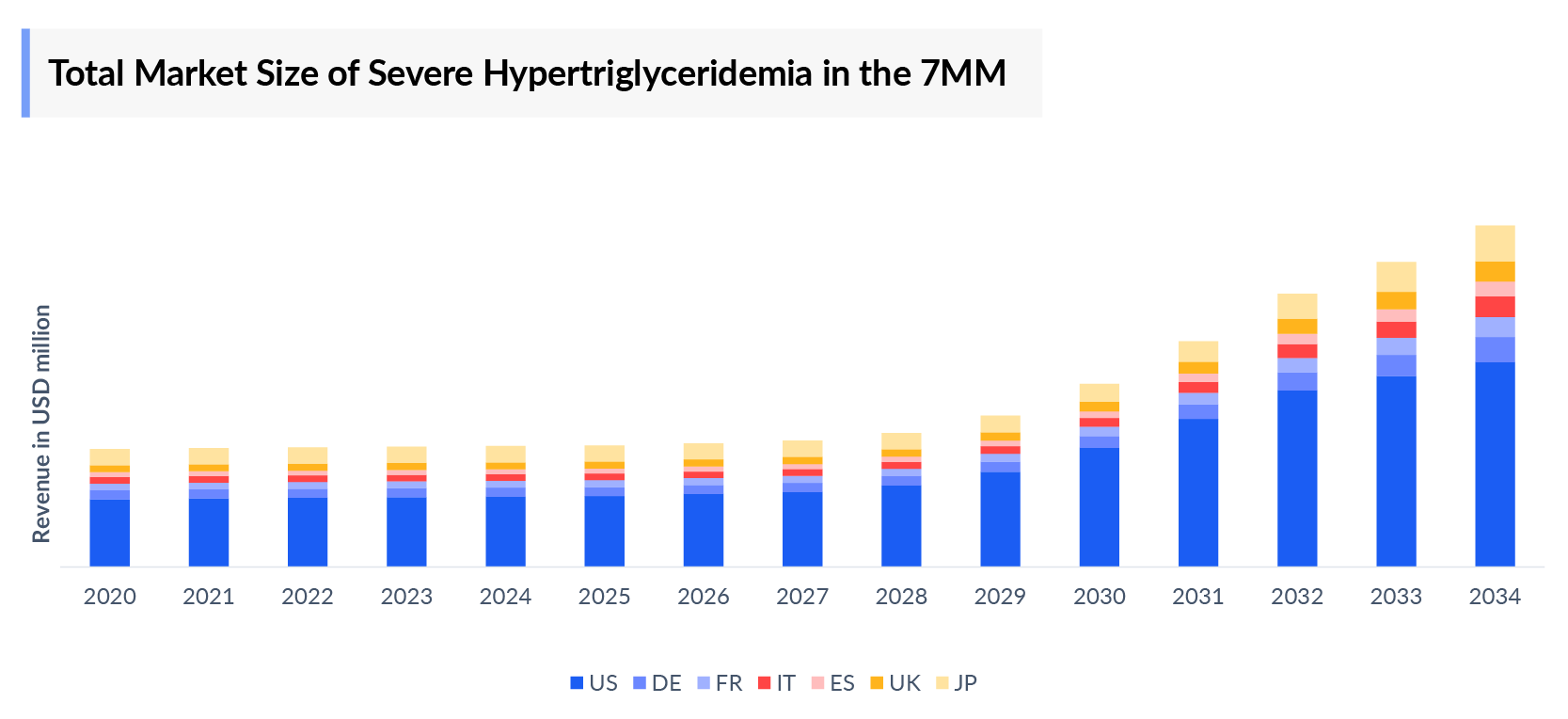 Severe Hypertriglyceridemia Market Outlook
Severe Hypertriglyceridemia Market Outlook
SHTG remains challenging to manage, with traditional treatments showing limited success in significantly lowering triglyceride levels or reducing the risk of acute pancreatitis, the most severe complication of SHTG. While dietary intervention is fundamental in treating primary hypertriglyceridemia, it often fails to achieve triglyceride levels below 500 mg/dl, the threshold for AP prevention. Conventional pharmacologic options—such as statins, fibrates, niacin, and omega-3 fatty acids—provide only modest reductions in triglyceride levels and lack compelling evidence of AP prevention. These treatments require a functional lipolytic pathway, making them less effective in Familial Chylomicronemia Syndrome (FCS), where this pathway is absent. This underscores a significant unmet need in the Severe Hypertriglyceridemia Therapeutics Market for more effective therapies that can target both triglyceride reduction and AP prevention.
There are three key omega-3 fatty acids: EPA, DHA, and ALA. EPA and DHA are primarily found in fish, while ALA comes from plants but converts minimally to EPA and DHA in the body. VASCEPA (icosapent ethyl), a purified form of EPA, is FDA-approved for SHTG and cardiovascular risk reduction. However, it only modestly lowers triglycerides and carries risks like atrial fibrillation and bleeding. In contrast to the marketed treatments, new therapeutic options have emerged, including antisense oligonucleotides and small interfering RNA (siRNA) targeting ApoC3, as well as recombinant proteins and small molecules. These novel approaches offer promising alternatives for more effective management of severe hypertriglyceridemia.
- The launch of Severe Hypertriglyceridemia Emerging Therapies, such as Olezarsen (Ionis Pharmaceuticals), ARO-APOC3 (Arrowhead Pharmaceuticals, Inc.), and Pegozafermin (89bio, Inc.) are expected to create a positive impact on the market.
- The Severe Hypertriglyceridemia Market Size in the 7MM was around USD 1,400 million in 2023. This is estimated to increase by 2034.
- Among the 7MM, the US consistently captured the highest market of about USD 1,300 million in 2023, which is anticipated to grow during the forecast period.
- Among EU4 and the UK, the highest Severe Hypertriglyceridemia Drugs Market Share was found in Germany which was estimated to be nearly USD 10 million of the market share in EU4 and the UK in 2023.
- Among the forecasted emerging therapies, Plozasiran is expected to capture the highest market in the 7MM by 2034.
Severe Hypertriglyceridemia Drugs Uptake
This section focuses on the rate of uptake of the potential Severe Hypertriglyceridemia drugs expected to be launched in the market during the study period 2020-2034. Olezarsen is an RNA-targeted investigational LIgand Conjugated Antisense (LICA) medicine developed by Ionis Pharmaceuticals, anticipated to enter the market in 2026. Olezarsen is anticipated to take medium uptake in the SHTG.
Severe Hypertriglyceridemia Pipeline Development Activities
The Severe Hypertriglyceridemia pipeline segment report provides insights into Severe Hypertriglyceridemia clinicla trials within Phase III, and and Phase II. It also analyzes key Severe Hypertriglyceridemia Companies involved in developing targeted therapeutics. The Severe Hypertriglyceridemia pipeline segment report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for Severe Hypertriglyceridemia emerging therapies.
Latest KOL- Views on Severe Hypertriglyceridemia
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on SHTG's evolving Severe Hypertriglyceridemia treatment market landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, include KOL from Southern California Permanente Medical Group, Centro de Salud Universitario Pinto, University of Glasgow, and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights, however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or SHTG market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Severe Hypertriglyceridemia Market and the unmet needs.
Severe Hypertriglyceridemia Therapeutics Market: Qualitative Analysis
We perform Qualitative and Severe Hypertriglyceridemia Therapeutics Market Intelligence analysis using various approaches, such as SWOT analysis, and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis is done to analyze multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Severe Hypertriglyceridemia Therapeutics Market Access and Reimbursement
SHTG is a lipid abnormality highly prevalent worldwide, specifically in the US adult population. FCS and MCM cause. Diet remains the mainstay of treatment of any form of primary HTG. However, it is often not sufficient to bring patients to TG levels of < 500 mg/dL, the generally accepted threshold for the prevention of acute pancreatitis. But besides this non-pharmacological approach, the pharmacological approach involves the uptake of therapies like fibrates, EPA, niacin, statin, and other drugs.
Besides these off-label medications, there is only one approved drug for SHTG, i.e., VASCEPA (icosapent ethyl), developed by Amarin Corporation. It is a small molecule that reduces hepatic VLDL-TG synthesis and enhances the TG clearance from circulating the VLDL particles. It was initially approved in the US in 2012 for SHTG and late in some parts of Europe, like Spain.
VASCEPA savings card
There are two ways in which patients can be saved. One is that physicians can e-prescribe VASCEPA to BlinkRx, an online prescription service that automatically applies the lowest available price after applying available discounts. The other one is to get a savings card. As per the VASCEPA savings card, eligible patients can pay as little as USD 9 for 90 days with the VASCEPA Savings Card. The card is available through BlinkRx, an online prescription service that seeks to find the lowest price. The patients (commercially insured patients) can pay as low as USD 0 for 90 days for eligible, and patients waiting on prior authorization approval can get a free 30-day fill. The lowest available cash price for patients is not covered by insurance.
The Severe Hypertriglyceridemia Therapeutics Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Severe Hypertriglyceridemia Market Report Scope
- The Severe Hypertriglyceridemia therapeutics market report covers a segment of key events, an executive summary, a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies along with the elaborative profiles of late-stage and prominent therapies will have an impact on the current Severe Hypertriglyceridemia treatment market landscape.
- A detailed review of the Severe Hypertriglyceridemia therapeutics market; historical and forecasted Severe Hypertriglyceridemia treatment market size, Severe Hypertriglyceridemia drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Severe Hypertriglyceridemia therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Severe Hypertriglyceridemia market.
Severe Hypertriglyceridemia Market Report Insights
- Patient-based Severe Hypertriglyceridemia Market Forecasting
- Severe Hypertriglyceridemia Therapeutics Approaches
- Severe Hypertriglyceridemia Pipeline Drugs Analysis
- Severe Hypertriglyceridemia Market Size and Trends
- Existing and future Severe Hypertriglyceridemia Market Opportunity
Severe Hypertriglyceridemia Market Report Key Strengths
- 11 Years Severe Hypertriglyceridemia Market Forecast
- 7MM Coverage
- SHTG Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Severe Hypertriglyceridemia Drugs Uptake
- Key Severe Hypertriglyceridemia Market Forecast Assumptions
Severe Hypertriglyceridemia Market Report Assessment
- Current Severe Hypertriglyceridemia Treatment Market Practices
- Severe Hypertriglyceridemia Unmet Needs
- Severe Hypertriglyceridemia Pipeline Drugs Analysis Profiles
- Severe Hypertriglyceridemia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Severe Hypertriglyceridemia Market Drivers
- Severe Hypertriglyceridemia Market Barriers
Key Questions Answered in the Severe Hypertriglyceridemia Market Report
- What was the Severe Hypertriglyceridemia Treatment Market Size, the Severe Hypertriglyceridemia market size by therapies, Severe Hypertriglyceridemia drugs market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of SHTG?
- What are the disease risks, burdens, and unmet needs of SHTG? What will be the growth opportunities across the 7MM concerning the patient population with SHTG?
- Who is the major competitor of VASCEPA/VAZKEPA in the market?
- Which class is performed better and generate highest revenue in 2024?
- What are the current options for the treatment of SHTG? What are the current guidelines for treating SHTG in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy the Severe Hypertriglyceridemia Market Report
- The Severe Hypertriglyceridemia Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the SHTG Market.
- Insights on patient burden/disease Severe Hypertriglyceridemia Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- To understand the existing Severe Hypertriglyceridemia drugs market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Severe Hypertriglyceridemia drugs market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Severe Hypertriglyceridemia Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles


-epidemiology.png&w=256&q=75)


