Systemic Sclerosis-associated Interstitial Lung Disease Market
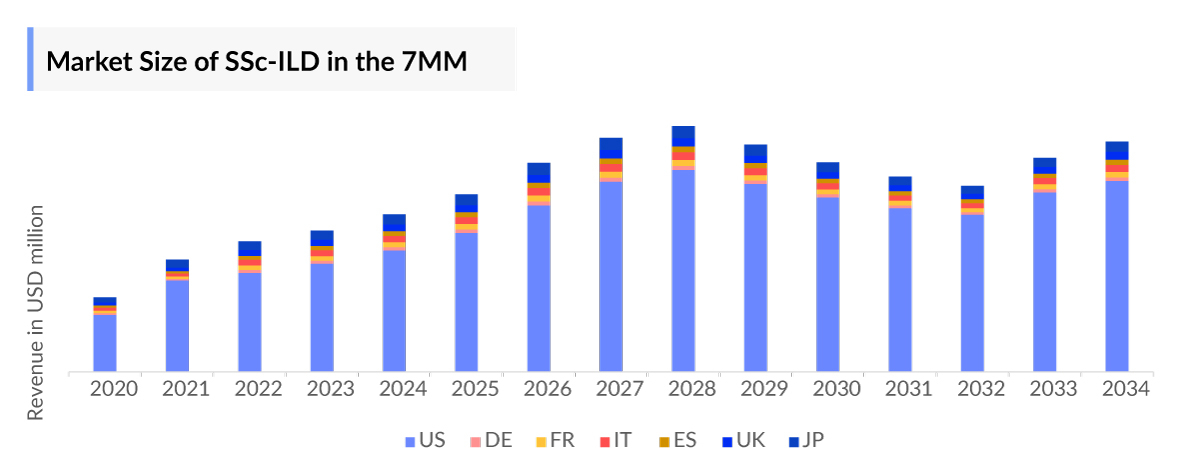
- The Systemic Sclerosis associated Lung Disease Market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of one-time gene therapies, and raised awareness.
- Systemic Sclerosis (SSc) is a disease characterized by fibrosis, vasculopathy, and inflammation that may affect different organs and systems, with severe prognostic implications. When SSc manifest at the lung level, the pulmonary disease may manifest as interstitial lung disease (ILD) and/or pulmonary arterial hypertension (PAH).
- Patients with SSc have a high probability of developing ILD as a complication. This combination of SSc-ILD can cause lung inflammation and scarring that lead to breathing failure.
- The United States accounts for the largest Systemic Sclerosis associated Lung Disease Market Size, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- The predominance of SSc-ILD cases in females in the US is due to the higher prevalence of systemic sclerosis in women, which is likely to be driven by complex genetic and hormonal factors.
- Severity-specific prevalence data suggests that the cases of the moderate type are much higher than the severe type of SSc-ILD.
Request for Unlocking the Sample Page of the "Systemic Sclerosis associated Lung Disease Treatment Market"
DelveInsight's “Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Systemic Sclerosis-associated Interstitial Lung Disease, historical and forecasted epidemiology as well as the Systemic Sclerosis-associated Interstitial Lung Disease market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Systemic Sclerosis-associated Interstitial Lung Disease Treatment Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Systemic Sclerosis-associated Interstitial Lung Disease Market Size from 2020 to 2034. The report also covers current Systemic Sclerosis-associated Interstitial Lung Disease treatment market practices/algorithms and Systemic Sclerosis associated Lung Disease unmet needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Systemic Sclerosis associated Lung Disease Epidemiology |
Segmented by:
|
|
Systemic Sclerosis associated Lung Disease Companies |
|
|
Systemic Sclerosis associated Lung Disease Drugs |
|
|
Systemic Sclerosis associated Lung Disease Therapeutics Market |
Segmented by:
|
|
Systemic Sclerosis associated Lung Disease Market Analysis |
|
Systemic Sclerosis associated Lung Disease Treatment Market: Overview
Systemic Sclerosis (SSc) is characterized by fibrosis, vasculopathy, and inflammation, affecting multiple organs. Pulmonary involvement can lead to interstitial lung disease (ILD) and/or pulmonary arterial hypertension (PAH). While the exact cause of SSc-ILD is unclear, environmental, immune, and genetic factors are implicated. SSc patients are at high risk of developing ILD, which can result in lung inflammation and scarring, leading to respiratory failure.
The diagnosis of Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) relies primarily on detecting interstitial lung disease (ILD) on high-resolution computed tomography (HRCT) of the chest in patients with known Systemic Sclerosis (SSc), while ruling out other causes of pulmonary parenchymal disease. A comprehensive clinical evaluation, including respiratory symptom assessment, is vital for all newly diagnosed SSc patients to identify potential lung involvement early. Early recognition is crucial as ILD significantly impacts morbidity and mortality in this population.
Further details related to diagnosis are provided in the report
Systemic Sclerosis-associated Interstitial Lung Disease Treatment
Treatment of SSc-ILD historically involved immunosuppressive agents, with hematopoietic stem cell transplantation (HSCT) and lung transplantation reserved for severe or rapidly progressive cases. The standard of care now includes cyclophosphamide and mycophenolate mofetil, albeit with modest improvements in forced vital capacity (FVC). Nintedanib and tocilizumab, approved by the FDA, are also used to slow pulmonary function decline in SSc-ILD patients.
Systemic Sclerosis-associated Interstitial Lung Disease Epidemiology
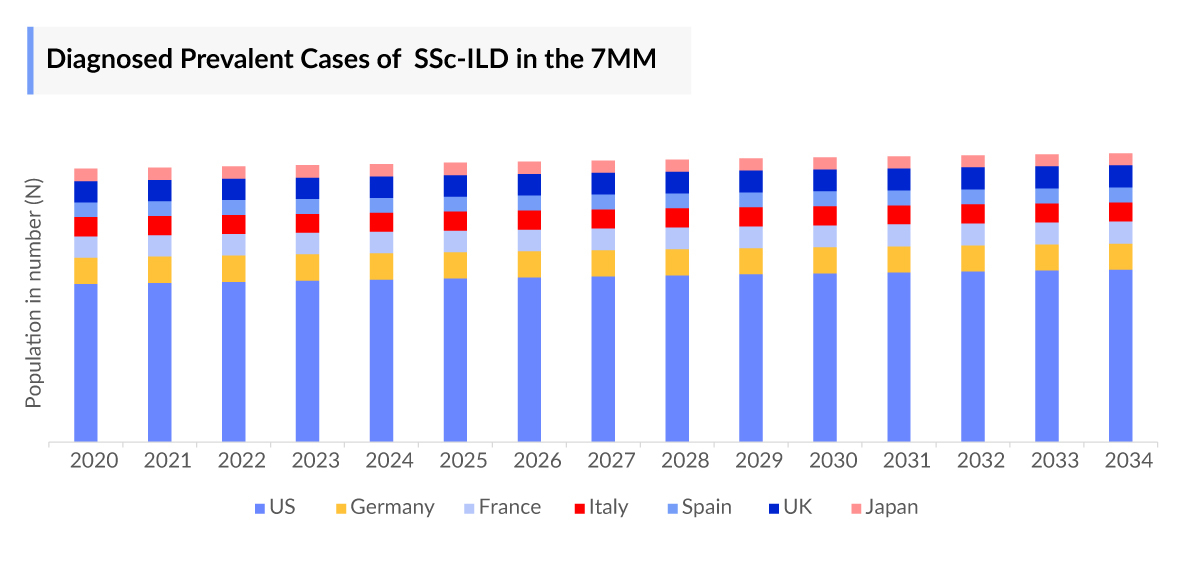
The Systemic Sclerosis-associated Interstitial Lung Disease epidemiology chapter in the report provides historical as well as forecasted epidemiology in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Systemic Sclerosis-associated Interstitial Lung Disease epidemiology is segmented with detailed insights into Total Diagnosed Prevalent Cases, Age-specific Diagnosed Prevalent Cases, Gender-specific Diagnosed Prevalent Cases, Severity-specific Diagnosed Prevalent Cases of Systemic Sclerosis-associated Interstitial Lung Disease.
- In 2023, among the EU4 and the UK, the United Kingdom had the highest diagnosed prevalent cases of SSc-ILD, followed by Italy and France. On the other hand, Germany had the lowest diagnosed prevalent cases of SSc-ILD in the same year.
- In 2023, as per Delveinsight’s analysis, the highest number of patients diagnosed with SSc-ILD belongs to age group 65 and above, and lowest in 0-18 age group.
- In 2023, the maximum number of diagnosed prevalent SSc-ILD cases in the US were found with moderate severity stage.
Unlock comprehensive insights! Click Here to Purchase the Full Report @ Systemic Sclerosis associated Lung Disease Prevalence
Systemic Sclerosis-associated Interstitial Lung Disease Drug Chapters
The drug chapter segment of the Systemic Sclerosis-associated Interstitial Lung Disease drugs market report encloses a detailed analysis of Systemic Sclerosis-associated Interstitial Lung Disease marketed drugs and late-stage (Phase III and Phase II) Systemic Sclerosis associated Lung Disease pipeline drugs analysis. It also deep dives into the pivotal Systemic Sclerosis-associated Interstitial Lung Disease clinical trials details, recent and expected market approvals, patent details, the latest Systemic Sclerosis associated Lung Disease news, and recent deals and collaborations.
Systemic Sclerosis associated Lung Disease Marketed Drugs
- ACTEMRA/ROACTEMRA (tocilizumab): Roche
In March 2021, FDA approved ACTEMRA (tocilizumab) subcutaneous injection for slowing the rate of decline in pulmonary function in adult patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD). Tocilizumab is a monoclonal antibody that selectively binds to the interleukin-6 receptor and prevents IL-6 from binding to its receptor (IL-6R) on the liver, lung, and synovial fibroblasts. In the liver, IL-6 can increase the production of C-reactive protein (CRP), serum amyloid A, fibrinogen, and hepcidin and decrease albumin synthesis. These liver mediator effects can cause amyloidosis, cardiovascular events, edema, and anemia.
Get More Insights @ ACTEMRA Market
- OFEV (nintedanib): Boehringer Ingelheim
In 2019, FDA approved OFEV (nintedanib) as the first and only medicine to slow the rate of decline in pulmonary function in patients with systemic sclerosis associated interstitial lung disease (SSc-ILD). Ofev is already approved in the US for the treatment of patients living with idiopathic pulmonary fibrosis (IPF), and has been shown to slow IPF progression by reducing the annual rate of decline in lung function, as measured by forced vital capacity (FVC). Ofev’s active ingredient nintedanib is a tyrosine kinase inhibitor (TKI) that inhibits the growth factor receptors involved in the progress of pulmonary fibrosis. Growth factor receptors involved in the scarring of lung tissue include platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR) and vascular endothelial growth factor receptor (VEGFR).
For More Insights @ OFEV (Nintedanib) Market
Note: Detailed current therapies assessment will be provided in the full report of Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD)
Systemic Sclerosis associated Lung Disease Emerging Drugs
- GSK1550188/Belimumab (BENLYSTA): GlaxoSmithKline
Belimumab (BENLYSTA) being developed by GSK, is a fully human monoclonal antibody that prevents B cells from differentiating. The company has initiated a Phase III trial of belimumab for SSc-ILD in September 2023. In February 2023, the US FDA granted Orphan Drug Designation (ODD) to BENLYSTA to treat SSc.
- PRA023: Merck
PRA023 is a humanized IgG1 monoclonal antibody (mAb) with a dual mechanism of action, targeting both inflammation and fibrosis, which provides a strong rationale for advancing PRA023 into clinical trials. It is currently in development for treating Ulcerative colitis (UC), Crohn’s Disease (CD), and SSc-ILD. In January 2022, the FDA granted Fast Track Designation (FTD) for PRA023 to treat SSc-ILD. The drug is currently recruiting participants in Phase II as per clinical trials. However, in June 2023, Prometheus Biosciences has now become a subsidiary of Merck & Co., Inc.
|
Therapy Name |
Company Name |
ROA |
MOA |
Phases |
Any Special Status |
|
GSK1550188/Belimumab (BENLYSTA) |
GlaxoSmithKline |
SC |
Human monoclonal antibody |
III |
Orphan Drug Designation (ODD) |
|
PRA023 |
Merck |
SC |
Humanized IgG1 monoclonal antibody (mAb) |
II |
Fast Track Designation (FTD) |
Systemic Sclerosis-associated Interstitial Lung Disease Market Outlook
Key Systemic Sclerosis associated Lung Disease Companies, such as Prometheus Biosciences, Genentech, GlaxoSmithKline, Merck & Co, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD).
- The current Systemic Sclerosis associated Lung Disease pipeline consists of potential drugs including, but not limited to PRA023, belimumab (GSK1550188), and vixarelimab (KPL-716).
- Among EU4 and the UK, UK constituted the highest Systemic Sclerosis associated Lung Disease Market Size in 2023.
Systemic Sclerosis-associated Interstitial Lung Disease Drugs Uptake
This section focuses on the uptake rate of potential Systemic Sclerosis associated Lung Disease drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Systemic Sclerosis associated Lung Disease Companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Systemic Sclerosis-associated Interstitial Lung Disease Pipeline Development Activities
The Systemic Sclerosis associated Lung Disease pipeline segment provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Systemic Sclerosis associated Lung Disease companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Systemic Sclerosis associated Lung Disease pipeline segment covers information on collaborations, acquisitions and mergers, licensing, and patent details for Systemic Sclerosis-associated Interstitial Lung Disease emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Systemic Sclerosis associated Lung Disease Treatment Drugs
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving Systemic Sclerosis associated Lung Disease treatment market landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Systemic Sclerosis-associated Interstitial Lung Disease. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Systemic Sclerosis associated Lung Disease drug market and the Systemic Sclerosis associated Lung Disease unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Systemic Sclerosis associated Lung Disease Therapeutics Market Access and Reimbursement
The Systemic Sclerosis associated Lung Disease drug market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Systemic Sclerosis associated Lung Disease Therapeutics Market Size Report Scope
- The Systemic Sclerosis associated Lung Disease Therapeutics Market Report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the Systemic Sclerosis associated Lung Disease epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Systemic Sclerosis-associated Interstitial Lung Disease drug market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Systemic Sclerosis associated Lung Disease Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Systemic Sclerosis-associated Interstitial Lung Disease market
Systemic Sclerosis-associated Interstitial Lung Disease Therapeutics Market Report Insights
- Patient-based Systemic Sclerosis associated Lung Disease Market Forecast
- Therapeutic Approaches
- Systemic Sclerosis associated Lung Disease Pipeline Drugs Analysis
- Systemic Sclerosis associated Lung Disease Market Size
- Systemic Sclerosis associated Lung Disease Market Trends
- Existing and future Systemic Sclerosis associated Lung Disease Drugs Market Opportunity
Systemic Sclerosis-associated Interstitial Lung Disease Therapeutics Market Report Key Strengths
- 11 Years- Systemic Sclerosis associated Lung Disease Market Forecast
- 7MM Coverage
- Systemic Sclerosis-associated Interstitial Lung Disease Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Systemic Sclerosis associated Lung Disease Drugs Uptake
- Key Systemic Sclerosis associated Lung Disease Market Forecast Assumptions
Systemic Sclerosis-associated Interstitial Lung Disease Treatment Market Report Assessment
- Current Systemic Sclerosis associated Lung Disease Treatment Market Practices
- Systemic Sclerosis associated Lung Disease Unmet Needs
- Systemic Sclerosis associated Lung Disease Pipeline Product Profiles
- Systemic Sclerosis associated Lung Disease Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM Systemic Sclerosis-associated Interstitial Lung Disease treatment market?
- What was the Systemic Sclerosis-associated Interstitial Lung Disease Therapeutics Market Size, the Systemic Sclerosis-associated Interstitial Lung Disease Treatment Market Size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the Systemic Sclerosis associated Lung Disease market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the Systemic Sclerosis associated Lung Disease treatment?
- How many companies are developing therapies for the treatment of Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD)?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The Systemic Sclerosis associated Lung Disease Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Systemic Sclerosis-associated Interstitial Lung Disease Drugs Market.
- Insights on patient burden/disease Systemic Sclerosis-associated Interstitial Lung Disease Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Systemic Sclerosis associated Lung Disease drug market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Systemic Sclerosis associated Lung Disease Companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Systemic Sclerosis associated Lung Disease unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles:-

.jpg)