Urea Cycle Disorders Market
- The Urea Cycle Disorders Market is expected to strengthen as awareness of the disease increases and more effective interventions are being developed.
- The leading Urea Cycle Disorders Companies such as Acer Therapeutics, Dimension Therapeutics, Callitas Therapeutics, Poseida Therapeutics, Promethera Biosciences, Arcturus Therapeutics, Kaleido Biosciences, Akaza Biopharma, Evox Therapeutics, Dipharma SA, Sana Biotechnology, Aeglea BioTherapeutics, Erytech, and others.
Request for unlocking the CAGR of the Urea Cycle Disorders Market
Factors affecting the Urea Cycle Disorder Market Growth
Rising Awareness and Improved Diagnosis of Rare Metabolic Disorders
Growing clinical awareness and improved screening programs for inborn errors of metabolism, especially neonatal screening for ammonia levels and genetic testing, have led to earlier and more accurate identification of Urea Cycle Disorders. This rise in diagnosis rates is significantly boosting market demand for disease management solutions.
Advancements in Genetic and Molecular Diagnostics
The availability of next-generation sequencing (NGS), enzyme assays, and carrier screening programs has improved detection of mutations in UCD-related genes such as OTC, ASS1, ASL, and CPS1. These technologies enhance diagnostic precision and facilitate early intervention, which supports market growth.
Growing Availability of Targeted Therapies
The market is witnessing significant progress with the introduction of ammonia-scavenging agents like Ravicti (glycerol phenylbutyrate), Buphenyl (sodium phenylbutyrate), and Ammonul (sodium benzoate and sodium phenylacetate). Their widespread use in chronic management has improved survival rates and patient quality of life, driving market expansion.
Increasing Research and Development Activities
Ongoing research into gene therapy, enzyme replacement therapy, and mRNA-based treatments is opening new therapeutic avenues. Companies are investing heavily in clinical trials to develop long-term curative approaches, reflecting robust pipeline activity in the UCD treatment space.
Supportive Government Policies and Orphan Drug Incentives
Regulatory bodies such as the FDA and EMA have granted orphan drug designations, priority reviews, and market exclusivity benefits for UCD therapies. These incentives encourage innovation and attract biopharmaceutical companies to invest in rare disease research.
Growing Newborn Screening Programs and Early Intervention Initiatives
Expansion of newborn metabolic screening programs across developed markets, coupled with improved physician education, has led to earlier diagnosis and timely initiation of treatment, reducing mortality and boosting therapeutic demand.
DelveInsight's "Urea Cycle Disorders Market Insights, Epidemiology, and Market Forecast-2034" report delivers an in-depth understanding of the Urea Cycle Disorders, historical and forecasted epidemiology as well as the Urea Cycle Disorders market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
The Urea Cycle Disorders Treatment Market report provides current treatment practices, emerging drugs, Urea Cycle Disorders market share of the individual therapies, current and forecasted Urea Cycle Disorders market Size from 2020 to 2034 segmented by seven major markets. The Report also covers current Urea Cycle Disorders treatment market practice/algorithm, market drivers, market barriers and Urea Cycle Disorders unmet needs to curate the best of the opportunities and assesses the underlying potential of the Urea Cycle Disorders Drugs Market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Urea Cycle Disorders Drugs Market |
|
|
Urea Cycle Disorders Market Size | |
|
Urea Cycle Disorders Companies |
|
Urea Cycle Disorders Treatment Market
The Urea Cycle Disorders Treatment Market is a critical segment of the pharmaceutical industry dedicated to addressing a group of rare genetic disorders known as urea cycle disorders (UCDs). These disorders impair the body's ability to effectively process nitrogen waste, leading to a toxic accumulation of ammonia in the bloodstream. The market encompasses a range of innovative therapies and interventions designed to manage and alleviate the symptoms of UCDs, ultimately improving patients' quality of life. The DelveInsight’s Urea Cycle Disorders market report gives a thorough understanding of the Urea Cycle Disorders by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis, and treatment.
Urea Cycle Disorders Diagnosis
Diagnosing urea cycle disorders involves a comprehensive approach aimed at identifying these rare genetic conditions that disrupt the body's ability to eliminate ammonia, a toxic byproduct of protein metabolism. This segment of the report covers the detailed diagnostic methods or tests for Urea Cycle Disorders.
Urea Cycle Disorders Treatment
The treatment of Urea Cycle Disorders (UCDs) revolves around managing the buildup of ammonia in the body, a toxic byproduct that accumulates due to a deficiency in the urea cycle enzymes. The primary goal is to reduce ammonia levels and prevent its detrimental effects on the central nervous system. This is typically achieved through a combination of dietary interventions and medications. It covers the details of conventional and current medical therapies available in the Urea Cycle Disorders market for the treatment of the condition. It also provides Urea Cycle Disorders treatment algorithms and guidelines in the United States, Europe, and Japan.
Recent Developments in the Urea Cycle Disorders Treatment Market Landscape
- In March 2025, iECURE reported data from the OTC-HOPE Phase I/II trial showing that the first infant treated with ECUR-506 achieved a complete clinical response, with signs of restored Ornithine Transcarbamylase (OTC) enzyme activity and urea cycle function.
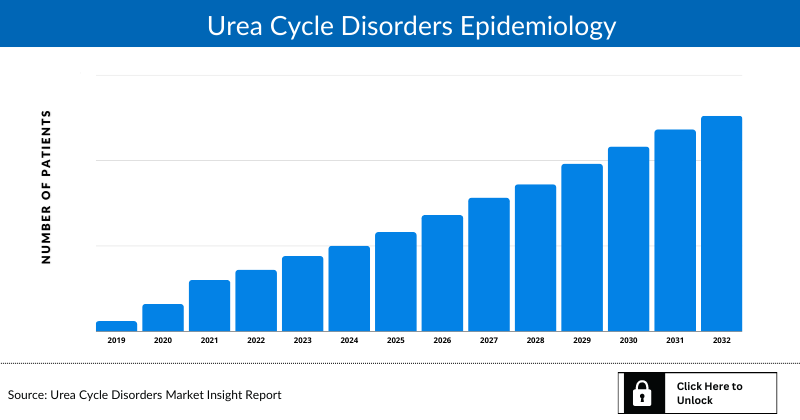
Urea Cycle Disorders Epidemiology
The Urea Cycle Disorders epidemiology section provides insights into the historical and current Urea Cycle Disorders patient pool and forecasted trends for individual seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the Urea Cycle Disorders market report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Urea Cycle Disorders Epidemiology Segmentation
- Urea Cycle Disorders Type-Specific Diagnosed Prevalent Cases
- Urea Cycle Disorders Onset-Specific Diagnosed Prevalent Cases
- Total Urea Cycle Disorders Diagnosed Prevalent Cases
- Urea Cycle Disorders Onset-specific Diagnosed Prevalent Cases
- Urea Cycle Disorders Treated Cases
Key Findings from Urea Cycle Disorders Epidemiological Analyses and Forecast
The disease epidemiology covered in the report provides historical as well as forecasted Urea Cycle Disorders Prevalence scenario in the 7MM covering the United States, EU5 countries (Germany, Spain, Italy, France, and the United Kingdom), and Japan from 2020 to 2034.
Country Wise- Urea Cycle Disorders Epidemiology
The epidemiology segment also provides the Urea Cycle Disorders epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan.
Urea Cycle Disorders Drugs Analysis
The drug chapter segment of the Urea Cycle Disorders treatment market report encloses the detailed analysis of Urea Cycle Disorders marketed drugs and late-stage (Phase-III and Phase-II) Urea Cycle Disorders pipeline drugs analysis. It also helps to understand the Urea Cycle Disorders clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Urea Cycle Disorders Marketed Drugs
The Urea Cycle Disorders therapeutics market report provides the details of the marketed products/off-label treatments available for Urea Cycle Disorders treatment.
Urea Cycle Disorders Emerging Drugs
-
DTX301: Ultragenyx Pharmaceutical
DTX301 is designed by Ultragenyx Pharmaceutical to deliver OTC gene expression in a durable fashion, with the goal of preventing or reducing the occurrence of complications associated with OTC deficiency. It has been granted ODD in the US, Europe and United Kingdom and Fast Track designation (FTD) in the US. Long term Phase I/II data demonstrated an acceptable safety profile and durable metabolic control and sustained responses. The company is conducting a Phase III (NCT05345171) trial to evaluate the efficacy of DTX301 on the improvement of OTC function by maintaining safe plasma ammonia levels with removal of dietary protein restriction and alternative pathway medication.
-
LUNAR-OTC (ARCT-810): Arcturus Therapeutics
LUNAR-OTC (ARCT-810), an mRNA therapy encoding OTC and delivered via lipid nanoparticles, has shown early promise in restoring urea cycle function and improving survival. It is currently in Phase II (NCT05526066) to assess safety, tolerability, and pharmacokinetics in adolescents and adults with OTC deficiency, with interim data expected in the first half of 2025.
In August 2024, ARCT-810, developed for the treatment of OTC deficiency, completed dosing of eight subjects in a Phase II double-blind, multiple-dose study. The program was further expanded with an open-label, multiple-dose study that began dosing in December 2024. ARCT-810 has received ODD, Rare Pediatric Disease Designation (RPDD), and Fast Track Designation (FTD) from the US FDA, along with ODD from the EMA.
-
ECUR-506 (formerly GTP-506): iECURE
ECUR-506 comprises two vectors, an ARCUS nuclease vector targeting gene editing in the wellcharacterized PCSK9 gene locus and a donor vector that inserts the desired functional OTC gene. ECUR506 is being studied in a Phase I/II (NCT06255782) OTC-HOPE study, the first clinical meganucleasebased in vivo gene insertion program. iECURE’s lead investigational candidate GTP-506 (also known as ECUR-506) for the treatment of neonatal OTC deficiency has been granted FTD, ODD, and RPDD by the US FDA, along with ODD by the European Commission.
Urea Cycle Disorders Market Outlook
The Urea Cycle Disorders market outlook of the report helps to build a detailed comprehension of the historic, current, and forecasted Urea Cycle Disorders market trends by analyzing the impact of current Urea Cycle Disorders therapies on the market, Urea Cycle Disorders unmet needs, drivers and barriers, and demand for better technology.
This segment gives a thorough detail of the Urea Cycle Disorders market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on the annual cost of therapy, inclusion, and exclusion criteria, mechanism of action, compliance rate, growing need of the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the market and view of the key opinion leaders. The calculated Urea Cycle Disorders market outlook data are presented with relevant tables and graphs to give a clear view of the market at first sight.
"According to DelveInsight, the Urea Cycle Disorders Treatment Market Size in 7MM is expected to witness a major change in the study period 2020-2034"
Key Findings
This section includes a glimpse of the Urea Cycle Disorders market in 7MM.
The United States Urea Cycle Disorders Market Outlook
This section provides the total Urea Cycle Disorders treatment market size and market size by therapies in the United States.
EU-5 Countries: Urea Cycle Disorders Market Outlook
The total Urea Cycle Disorders treatment market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom is provided in this section.
Japan Urea Cycle Disorders Market Outlook
The total Urea Cycle Disorders treatment market size and market size by therapies in Japan is also mentioned.
Urea Cycle Disorders Drugs Uptake
This section focuses on the rate of uptake of the potential Urea Cycle Disorders drugs recently launched in the Urea Cycle Disorders market or expected to get launched in the market during the study period 2020-2034. The analysis covers Urea Cycle Disorders market uptake by drugs; patient uptake by therapies; and sales of each drug. Urea Cycle Disorders Drugs Uptake helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allow the comparison of the drugs on the basis of Urea Cycle Disorders market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Urea Cycle Disorders Clinical Trial Analysis
The Urea Cycle Disorders pipeline segment provides insights into different therapeutic candidates in Phase II, and Phase III stage. It also analyses Urea Cycle Disorders Companies involved in developing targeted therapeutics.
Urea Cycle Disorders Pipeline Development Activities
The Urea Cycle Disorders pipeline segment covers the detailed information of collaborations, acquisition, and merger, licensing, patent details, and other information for Urea Cycle Disorders emerging therapies.
Reimbursement Scenario
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we take reimbursement into consideration to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
Latest KOL- Views on Urea Cycle Disorders
To keep up with current Urea Cycle Disorders market trends, we take KOLs and SMEs ' opinion working in the Urea Cycle Disorders domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Urea Cycle Disorders market trends. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the Urea Cycle Disorders unmet needs.
Urea Cycle Disorders Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Urea Cycle Disorders Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Urea Cycle Disorders Treatment Market Report Scope
- The Urea Cycle Disorders therapeutics market report covers the descriptive overview, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
- Comprehensive insight has been provided into the Urea Cycle Disorders epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Urea Cycle Disorders is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Urea Cycle Disorders therapeutics market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The patient-based Urea Cycle Disorders market forecasting report provides an edge while developing business strategies, by understanding trends shaping and driving the global Urea Cycle Disorders drugs market
Urea Cycle Disorders Treatment Market Report Highlights
- In the coming years, the Urea Cycle Disorders therapeutics market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The Urea Cycle Disorders companies and academics are working to assess challenges and seek opportunities that could influence Urea Cycle Disorders R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major Urea Cycle Disorders companies are involved in developing therapies for Urea Cycle Disorders. The launch of emerging therapies will significantly impact the Urea Cycle Disorders market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Urea Cycle Disorders
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed Urea Cycle Disorders clinical trials profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Urea Cycle Disorders Market Report Insights
- Patient-based Urea Cycle Disorders Market Forecasting
- Therapeutic Approaches
- Urea Cycle Disorders Pipeline Drugs Analysis
- Urea Cycle Disorders Market Size and Trends
- Urea Cycle Disorders Drugs Market Opportunities
- Impact of Upcoming Urea Cycle Disorders Therapies
Urea Cycle Disorders Market Report Key Strengths
- 10 Years Urea Cycle Disorders Market Forecast
- 7MM Coverage
- Urea Cycle Disorders Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Urea Cycle Disorders Drugs Market
- Urea Cycle Disorders Drugs Uptake
Urea Cycle Disorders Market Report Assessment
- Current Urea Cycle Disorders Treatment Market Practices
- Urea Cycle Disorders Unmet Needs
- Urea Cycle Disorders Pipeline Drugs Profiles
- Urea Cycle Disorders Therapeutics Market Attractiveness
- Urea Cycle Disorders Market Drivers
- Urea Cycle Disorders Market Barriers
Key Questions covered in the Urea Cycle Disorders Market
Urea Cycle Disorders Market Insights
- What was the Urea Cycle Disorders drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Urea Cycle Disorders market size as well as market size by therapies across the 7MM during the forecast period (2025-2034)?
- What are the key findings pertaining to the market across 7MM and which country will have the largest Urea Cycle Disorders market size during the forecast period (2025-2034)?
- At what CAGR, the Urea Cycle Disorders market is expected to grow by 7MM during the forecast period (2025-2034)?
- What would be the Urea Cycle Disorders market outlook across the 7MM during the forecast period (2025-2034)?
- What would be the Urea Cycle Disorders market growth till 2032, and what will be the resultant market Size in the year 2032?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Urea Cycle Disorders Epidemiology Insights
- What are the disease risk, burden, and regional/ethnic differences of the Urea Cycle Disorders?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What is the historical Urea Cycle Disorders patient pool in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What would be the forecasted patient pool of Urea Cycle Disorders in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- Where will be the growth opportunities in the 7MM with respect to the patient population pertaining to Urea Cycle Disorders?
- Out of all 7MM countries, which country would have the highest prevalent population of Urea Cycle Disorders during the forecast period (2025-2034)?
- At what CAGR the patient population is expected to grow in 7MM during the forecast period (2025-2034)?
Current Urea Cycle Disorders Treatment Market Scenario, Marketed Drugs and Emerging Therapies
- What are the current options for the Urea Cycle Disorders treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of Urea Cycle Disorders in the USA, Europe, and Japan?
- What are the Urea Cycle Disorders marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety and efficacy, etc.?
- How many companies are developing therapies for the treatment of Urea Cycle Disorders?
- How many therapies are in-development by each company for Urea Cycle Disorders treatment?
- How many are emerging therapies in mid-stage, and late stage of development for Urea Cycle Disorders treatment?
- What are the key collaborations (Industry - Industry, Industry - Academia), Mergers and acquisitions, licensing activities related to the Urea Cycle Disorders therapies?
- What are the recent novel therapies, targets, mechanisms of action and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Urea Cycle Disorders and their status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for Urea Cycle Disorders?
- What are the global historical and forecasted Urea Cycle Disorders Drugs Market?
Reasons to buy the Urea Cycle Disorders Market Report
- The patient-based Urea Cycle Disorders market forecasting report will help in developing business strategies by understanding trends shaping and driving the Urea Cycle Disorders drugs market
- To understand the future market competition in the Urea Cycle Disorders drugs market and Insightful review of the key market drivers and barriers
- Organize sales and marketing efforts by identifying the best opportunities for Urea Cycle Disorders in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom) and Japan
- Identification of strong upcoming players in the Urea Cycle Disorders drugs market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for Urea Cycle Disorders drugs market
- To understand the future market competition in the Urea Cycle Disorders drugs market
Stay updated with us for Related Articles