Wet Age-Related Macular Degeneration (Wet AMD) Market
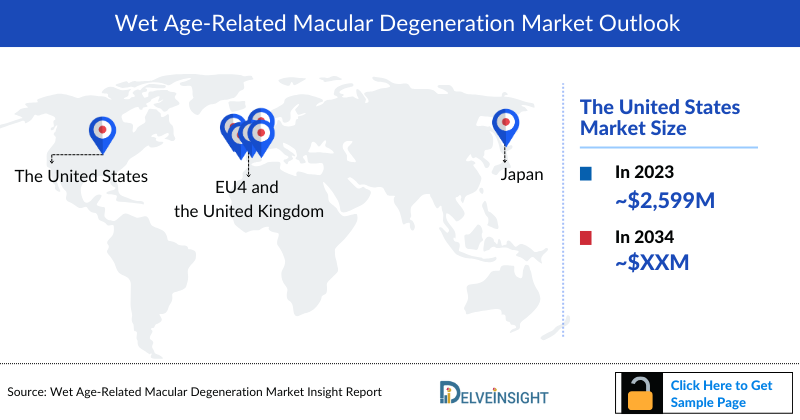
- In 2023, the market size of Wet Age-Related Macular Degeneration (Wet AMD) was highest in the US among the 7MM, accounting for approximately USD 2,599 million which is further expected to rise at a CAGR of 6.6% by 2034.
- In 2023, the prevalence of Age-Related Macular Degeneration (AMD) was highest in the US among the 7MM, accounting for nearly 18 million thousand cases.
- Various therapies are employed to treat Wet Age-Related Macular Degeneration (Wet AMD), currently approved therapies includes EYLEA (aflibercept), LUCENTIS (ranibizumab), BEOVU (brolucizumab), AVASTIN (bevacizumab), and Others. Among these, EYLEA had the highest market share in 2023, accounting for approximately USD 4,276 million in the 7MM, according to our analysis.
- The market is driven by the aging population, innovative therapies, and increased screening, while high costs, limited access, and treatment burden are key barriers. Reimbursement varies, with growing emphasis on value-based models. Unmet needs include longer-acting therapies, improved efficacy, and better early detection methods.
- The market size of Wet Age-Related Macular Degeneration (Wet AMD) in Japan was USD 1,163 million in 2023, which is expected to rise at a significant CAGR of 2.6% by 2034.
- The emerging drug Sozinibercept (OPT-302) and is expected to launch in the US by 2025, in EU4 and the UK by 2026, and in Japan by 2027, which has the potential to reduce the disease burden of Wet Age-Related Macular Degeneration (Wet AMD) in the forecasted years.
- Opthea Limited is currently conducting two pivotal Phase III trials, COAST and ShORe, to evaluate the safety and efficacy of OPT-302 (sozinibercept) in combination with standard-of-care anti-VEGF-A therapies for the treatment of wet age-related macular degeneration (AMD).
DelveInsight’s “Wet Age-Related Macular Degeneration (Wet AMD) – Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the Wet Age-Related Macular Degeneration (Wet AMD), historical and forecasted epidemiology as well as the Wet Age-Related Macular Degeneration (Wet AMD) market trends in the United States, EU4 and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Wet Age-Related Macular Degeneration (Wet AMD) market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Wet Age-Related Macular Degeneration (Wet AMD) market size from 2020 to 2034. The Report also covers current Wet Age-Related Macular Degeneration (Wet AMD) treatment practice, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Wet Age-Related Macular Degeneration Treatment Market
Wet Age-Related Macular Degeneration (Wet AMD) Overview
Wet Age-Related Macular Degeneration (Wet AMD) is a chronic eye disorder that results from the abnormal growth of blood vessels beneath the macula, a small area of the retina responsible for central vision. These vessels leak blood and fluid, causing rapid and severe vision loss. The main risk factors include age, genetic predisposition, and lifestyle factors like smoking. Wet AMD is typically more severe than its counterpart, dry AMD, and can lead to permanent central vision impairment.
Wet Age-Related Macular Degeneration (Wet AMD) Diagnosis
Diagnosing wet age-related macular degeneration (AMD) involves several steps. Initial evaluation includes a comprehensive eye exam with dilated fundus photography to visualize the retina and macula. Optical coherence tomography (OCT) is essential for identifying fluid or blood under the retina, indicative of wet AMD. Fluorescein angiography may also be used to detect leaking blood vessels in the macula. Symptoms prompting diagnosis typically include blurred vision, dark spots, or distorted lines in the central vision. Early detection is critical, as prompt treatment can slow disease progression and preserve vision. Patients with wet AMD require ongoing monitoring and care.
Further details related to diagnosis are provided in the report...
Wet Age-Related Macular Degeneration (Wet AMD) Treatment
Treatment for Wet-Age Related Macular Degeneration (Wet-AMD) primarily focuses on halting disease progression and preserving vision. The standard treatment involves the use of anti-VEGF (vascular endothelial growth factor) drugs such as Ranibizumab (Lucentis), Aflibercept (Eylea), and Brolucizumab (Beovu). These are administered via intravitreal injections to reduce new blood vessel growth and fluid leakage in the retina. Some patients may benefit from photodynamic therapy (PDT), which uses light-activated drugs to destroy abnormal blood vessels. Advances in gene therapy and implantable devices are also under investigation to provide longer-lasting treatment solutions and improve patient outcomes.
Further details related to treatment are provided in the report...
Wet Age-Related Macular Degeneration (Wet AMD) Epidemiology
As the market is derived using a patient-based model, the Wet Age-Related Macular Degeneration (Wet AMD) epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Total Prevalent Cases Of Age-Related Macular Degeneration (AMD), Diagnosed Prevalent Cases Of Age-Related Macular Degeneration (AMD), Type-Specific Diagnosed Prevalent Cases Of Age-Related Macular Degeneration (AMD), and Total Age-Specific Cases Of Wet-AMD in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
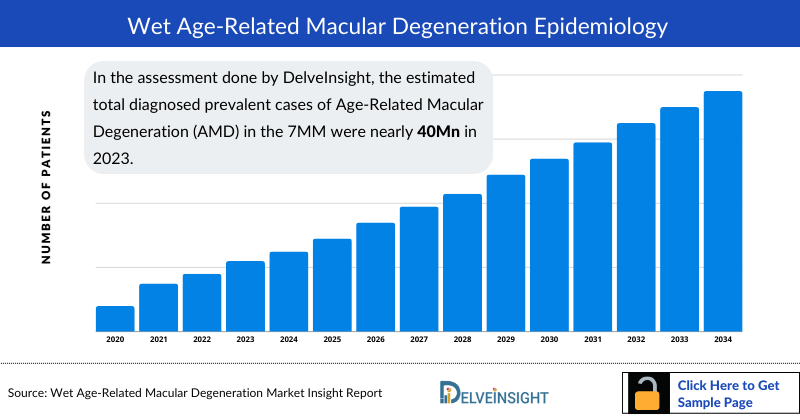
- In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of Age-Related Macular Degeneration (AMD) in the 7MM were nearly 40 million in 2023.
- The highest total diagnosed prevalent cases of Age-Related Macular Degeneration (AMD) were accounted by the US in 2023 (~14 million), which are expected to show a rise in the future.
- Among the European countries, Germany had the highest diagnosed prevalent cases of Age-Related Macular Degeneration (AMD) with ~6 million cases in 2023. On the other hand, Spain had the lowest prevalent population (~2 million cases).
- Japan had nearly 8 million total diagnosed prevalent cases of Age-Related Macular Degeneration (AMD) in 2023, accounting for approximately 21% in 7MM.
- Based on age-specific segmentation, the people in the age group of 70-79 were affected the most by Wet Age-Related Macular Degeneration (Wet AMD) in the US, accounting for approximately 406 thousand cases in 2023.
- The DelveInsight analysis indicates that in Japan, there are more number of cases of people with Dry AMD than people with Wet AMD, with approximately 90% of total cases and 10% of cases in 2023.
- Among the European countries, Germany had the highest diagnosed prevalent cases of Wet AMD with ~595 thousand cases, followed by France, which had prevalent population of ~389 thousand in 2023. On the other hand, Spain had the lowest prevalent population (~160 thousand cases).
- Japan had ~840 thousand total diagnosed prevalent cases of Wet Age-Related Macular Degeneration (Wet AMD) in 2023, accounting for approximately 21% in 7MM.
- In 2023, in the US, the age-specific diagnosed prevalent cases of Wet AMD were highest for age group 70–79 (~407 thousand), followed by 60–69 (~393 thousand), =80 (~304 thousand), and 50–59 (~252 thousand).
Wet Age-Related Macular Degeneration (Wet AMD) Recent Developments
- In August 2025, Outlook Therapeutics announced that the FDA issued a complete response letter (CRL) for its biologics license application for the treatment of wet age-related macular degeneration (wet AMD), indicating the application cannot be approved in its current form.
- In April 2025, Outlook Therapeutics announced that the U.S. FDA has received the resubmitted Biologics License Application (BLA) for ONS-5010 (bevacizumab-vikg), an investigational ophthalmic bevacizumab formulation for treating wet age-related macular degeneration (wet AMD).
Wet Age-Related Macular Degeneration (Wet AMD) Drug Chapters
The drug chapter segment of the Wet Age-Related Macular Degeneration (Wet AMD) report encloses a detailed analysis of Wet Age-Related Macular Degeneration (Wet AMD) off-label drugs and late-stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Wet Age-Related Macular Degeneration (Wet AMD) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Wet Age-Related Macular Degeneration Marketed Drugs
BEOVU (Brolucizumab): Novartis
BEOVU (Brolucizumab), developed by Novartis, is a potent anti-VEGF treatment used for Wet Age-Related Macular Degeneration (Wet-AMD). It works by inhibiting vascular endothelial growth factor, reducing abnormal blood vessel growth and fluid leakage in the retina. BEOVU offers a longer dosing interval, allowing up to 12-week dosing schedules.
EYLEA (aflibercept): Regeneron Pharmaceuticals
EYLEA, developed by Regeneron, is used to treat wet age-related macular degeneration (wet-AMD), diabetic eye disease, and other retinal issues. The drug, aflibercept, is an injectable treatment that slows vision loss by blocking abnormal blood vessel growth in the eye. EYLEA works as a soluble decoy receptor, inhibiting VEGF-A and PlGF to prevent abnormal blood vessel formation. The standard dosage is 2 mg every 4 weeks for the first 3 months, then every 8 weeks. Regeneron is currently testing less frequent dosing and higher doses in ongoing phase III trials.
EYLEA (aflibercept) received FDA approval in November 2011 for treating wet-AMD with a dosage of 2 mg every 4 weeks for the first 12 weeks, then every 8 weeks. It was approved by the European Commission in November 2012 and by Japan's MHLW in September 2012 for the same condition.
VABYSMO (faricimab): Roche/ Genentech, Inc.
VABYSMO (faricimab) is the first bispecific antibody designed for the eye. It targets and inhibits two signalling pathways linked to a number of vision-threatening retinal conditions by neutralizing angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A). Ang-2 and VEGF-A contribute to vision loss by destabilizing blood vessels, causing new leaky blood vessels to form and increasing inflammation. By blocking both pathways involving Ang-2 and VEGF-A, VABYSMO is designed to stabilize blood vessels.
VABYSMO is now approved in the European Union and nine other countries around the world, including the US, Japan, and the UK, for people living with "wet" age-related macular degeneration and diabetic macular edema.
Wet Age-Related Macular Degeneration Emerging Drugs
OPT-302: Opthea Limited
OPT-302 (sVEGFR-3) is the first ‘Trap’ inhibitor of VEGF-C and VEGF-D designed specifically for the eye. OPT-302 blocks the two members of the VEGF family which cause blood vessels to grow and leak. Aberrant blood vessel growth and vascular leakage are hallmarks of several eye diseases, including wet AMD and DME. In combination with anti-VEGF-A therapies, OPT-302 completely shuts-down VEGFR-2 and VEGFR-3 activity and targets mechanisms of resistance and suboptimal clinical response to existing therapies.
In July 2021, Opthea announced that FDA granted Fast Track designation for the company’s VEGF-C/-D ‘trap’ inhibitor, OPT-302, in combination with anti-VEGF-A therapy for the treatment of patients with wet age-related macular degeneration (AMD).
Currently the drug is being evaluated in two pivotal Phase III clinical trials (COAST; NCT04757636, and ShORe; NCT04757610) for use in the treatment of Wet AMD in combination with standard-of-care anti-VEGF-A monotherapies to improve overall efficacy and deliver superior vision gains compared to standard-of-care anti-VEGF-A agents.
KSI-501: Kodiak Sciences Inc.
KSI-501 is a novel anti-VEGF biologic designed to rapidly inhibit VEGF and provide extended durability of action to reduce the burden of frequent anti-VEGF injections. Delivering potent and sustained VEGF inhibition enables patient compliance, results in long-term efficacy, and improves visual acuity outcomes.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
OPT-302 |
Angiogenesis inhibitors; Vascular endothelial growth factor C inhibitors; Vascular endothelial growth factor D inhibitors |
Intravitreal Injection |
Opthea Limited |
III |
|
KSI-501 |
Interleukin 6 inhibitors; Vascular endothelial growth factors inhibitors |
Intravitreal Injection |
Kodiak Sciences Inc. |
III |
|
XXX |
Gene transference |
XXX |
XXX |
II |
Note: Detailed emerging therapies assessment will be provided in the final report of Wet Age-Related Macular Degeneration (Wet AMD)...
Wet Age-Related Macular Degeneration (Wet AMD) Market Outlook
Wet Age-Related Macular Degeneration (wet AMD) is a progressive eye condition characterized by the growth of abnormal blood vessels beneath the retina, which can lead to severe vision loss. Treatment aims to manage symptoms, slow disease progression, and preserve vision. The primary therapeutic approach for wet AMD is anti-vascular endothelial growth factor (anti-VEGF) therapy. These medications, including ranibizumab (LUCENTIS), aflibercept (EYLEA), and brolucizumab (BEOVU), work by inhibiting VEGF, a protein that promotes the growth of abnormal blood vessels. Administered via intravitreal injections, these drugs reduce fluid leakage and vascular growth, stabilizing or improving vision in many patients.
Another treatment option is photodynamic therapy (PDT), which involves intravenous administration of a light-sensitive drug, verteporfin (Visudyne), followed by the application of a low-level laser to the eye. PDT targets and destroys the abnormal blood vessels without causing significant damage to the surrounding retina. However, its use has declined with the advent of more effective anti-VEGF therapies.
In some cases, thermal laser photocoagulation might be employed to directly destroy the abnormal blood vessels, though this method is less common due to its potential to cause scarring and vision loss. Additionally, recent advancements include the development of sustained-release anti-VEGF delivery systems, such as port delivery systems, which aim to reduce the frequency of injections.
Overall, the choice of treatment depends on the specific characteristics of the AMD and the patient's response to initial therapies. Regular monitoring and individualized treatment plans are crucial to achieving the best outcomes and preserving vision for patients with wet AMD.
- The market size of Wet Age-Related Macular Degeneration (Wet AMD) in the 7MM was nearly USD 7,783 million in 2023, which is further anticipated to increase during the forecast period.
- The United States accounted for the highest market size of Wet Age-Related Macular Degeneration (Wet AMD) approximately 33% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the EU countries, Germany had the highest market size with nearly USD 1,313 million each in 2023, while Spain had the lowest market size for Wet Age-Related Macular Degeneration (Wet AMD) with USD ~ 353 million in 2023.
- With the expected launch of upcoming therapies, such as OPT-302, KSI-501, and RGX-314, among others, the total market size of Wet Age-Related Macular Degeneration (Wet AMD) is expected to show change in the upcoming years.
Wet Age-Related Macular Degeneration (Wet AMD) Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020–2034. For example, RGX-314 in the US is expected to be launched by 2026 with a peak share of 0.5%. RGX-314 is anticipated to take 6 years to peak with a medium uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Wet Age-Related Macular Degeneration (Wet AMD) Pipeline Development Activities
The report provides insights into different Wet Age-Related Macular Degeneration clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Wet Age-Related Macular Degeneration (Wet AMD) emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Wet Age-Related Macular Degeneration (Wet AMD) evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Cleveland Clinic – Cole Eye Institute, Cleveland, US; Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, US; Bascom Palmer Eye Institute/University of Miami Miller School of Medicine, Miami; Thomas Jefferson University, US; University of Louisville School of Medicine, Louisville, US; Department of Ophthalmology, University of Bonn, Germany; Department of Experimental Medicine, University Tor Vergata, Viale Oxford, Rome, Italy; Department of Ophthalmology, Kansai Medical University, Hirakata, Osaka, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Wet Age-Related Macular Degeneration (Wet AMD) market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Wet Age-Related Macular Degeneration Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Wet Age-Related Macular Degeneration (Wet AMD), explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Wet Age-Related Macular Degeneration (Wet AMD) market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Wet Age-Related Macular Degeneration (Wet AMD) market.
Wet Age-Related Macular Degeneration (Wet AMD) Report Insights
- Wet Age-Related Macular Degeneration Patient Population
- Wet Age-Related Macular Degeneration Therapeutic Approaches
- Wet Age-Related Macular Degeneration (Wet AMD) Pipeline Analysis
- Wet Age-Related Macular Degeneration (Wet AMD) Market Size and Trends
- Existing and Future Market Opportunity
Wet Age-Related Macular Degeneration (Wet AMD) Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Wet Age-Related Macular Degeneration (Wet AMD) Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Wet Age-Related Macular Degeneration Drugs Uptake
- Key Wet Age-Related Macular Degeneration Market Forecast Assumptions
Wet Age-Related Macular Degeneration (Wet AMD) Report Assessment
- Current Wet Age-Related Macular Degeneration Treatment Practices
- Wet Age-Related Macular Degeneration Unmet Needs
- Wet Age-Related Macular Degeneration Pipeline Product Profiles
- Wet Age-Related Macular Degeneration Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Wet Age-Related Macular Degeneration Market Drivers
- Wet Age-Related Macular Degeneration Market Barriers
Key Questions
- Wet Age-Related Macular Degeneration Market Insights
- What was the Wet Age-Related Macular Degeneration (Wet AMD) market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Wet Age-Related Macular Degeneration (Wet AMD) total market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Wet Age-Related Macular Degeneration (Wet AMD) market size during the forecast period (2024–2034)?
- At what CAGR, the Wet Age-Related Macular Degeneration (Wet AMD) market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Wet Age-Related Macular Degeneration (Wet AMD) market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Wet Age-Related Macular Degeneration (Wet AMD) market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Wet Age-Related Macular Degeneration Epidemiology Insights
- What is the disease risk, burden, and unmet needs of Wet Age-Related Macular Degeneration (Wet AMD)?
- What is the historical Wet Age-Related Macular Degeneration (Wet AMD) patient population in the United States, EU5 (Germany, France, Italy, Spain, and the UK), and Japan?
- What would be the forecasted patient population of Wet Age-Related Macular Degeneration (Wet AMD) at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Wet Age-Related Macular Degeneration (Wet AMD)?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Wet Age-Related Macular Degeneration (Wet AMD) during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Wet Age-Related Macular Degeneration Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Wet Age-Related Macular Degeneration (Wet AMD) along with the approved therapy?
- What are the current treatment guidelines for the treatment of Wet Age-Related Macular Degeneration (Wet AMD) in the US, Europe, And Japan?
- What are the Wet Age-Related Macular Degeneration (Wet AMD) marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many companies are developing therapies for the treatment of Wet Age-Related Macular Degeneration (Wet AMD)?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Wet Age-Related Macular Degeneration (Wet AMD)?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Wet Age-Related Macular Degeneration (Wet AMD) therapies?
- What are the recent therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Wet Age-Related Macular Degeneration (Wet AMD) and their status?
- What are the key designations that have been granted for the emerging therapies for Wet Age-Related Macular Degeneration (Wet AMD)?
- What are the 7MM historical and forecasted market of Wet Age-Related Macular Degeneration (Wet AMD)?
Reasons to Buy Wet Age-Related Macular Degeneration Market Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Wet Age-Related Macular Degeneration (Wet AMD) Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
1. What is the forecast period covered in the report?
The Wet Age-Related Macular Degeneration (Wet AMD) Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the Wet Age-Related Macular Degeneration (Wet AMD) market?
The Wet Age-Related Macular Degeneration (Wet AMD) market is quite robust. The major layers are REGENXBIO, Kodiak Sciences Inc., Opthea Limited, and others which are currently developing drugs for the treatment of Wet Age-Related Macular Degeneration (Wet AMD).
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as prevalent cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the Wet Age-Related Macular Degeneration (Wet AMD) market?
The increase in diagnosed prevalent cases of Wet Age-Related Macular Degeneration (Wet AMD) and the launch of emerging therapies are attributed to be the key drivers for increasing the Wet Age-Related Macular Degeneration (Wet AMD) market.
5. What is the expected impact of emerging therapies or advancements in Wet Age-Related Macular Degeneration (Wet AMD) treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the Wet Age-Related Macular Degeneration (Wet AMD) treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the Wet Age-Related Macular Degeneration (Wet AMD) market.

 Market.png)