Cutaneous T-cell Lymphoma market size is projected to grow at a CAGR of 5.0% by 2034
Get a Sneak Peek at the Latest cutaneous t cell lymphoma ctcl market size and forecast Report
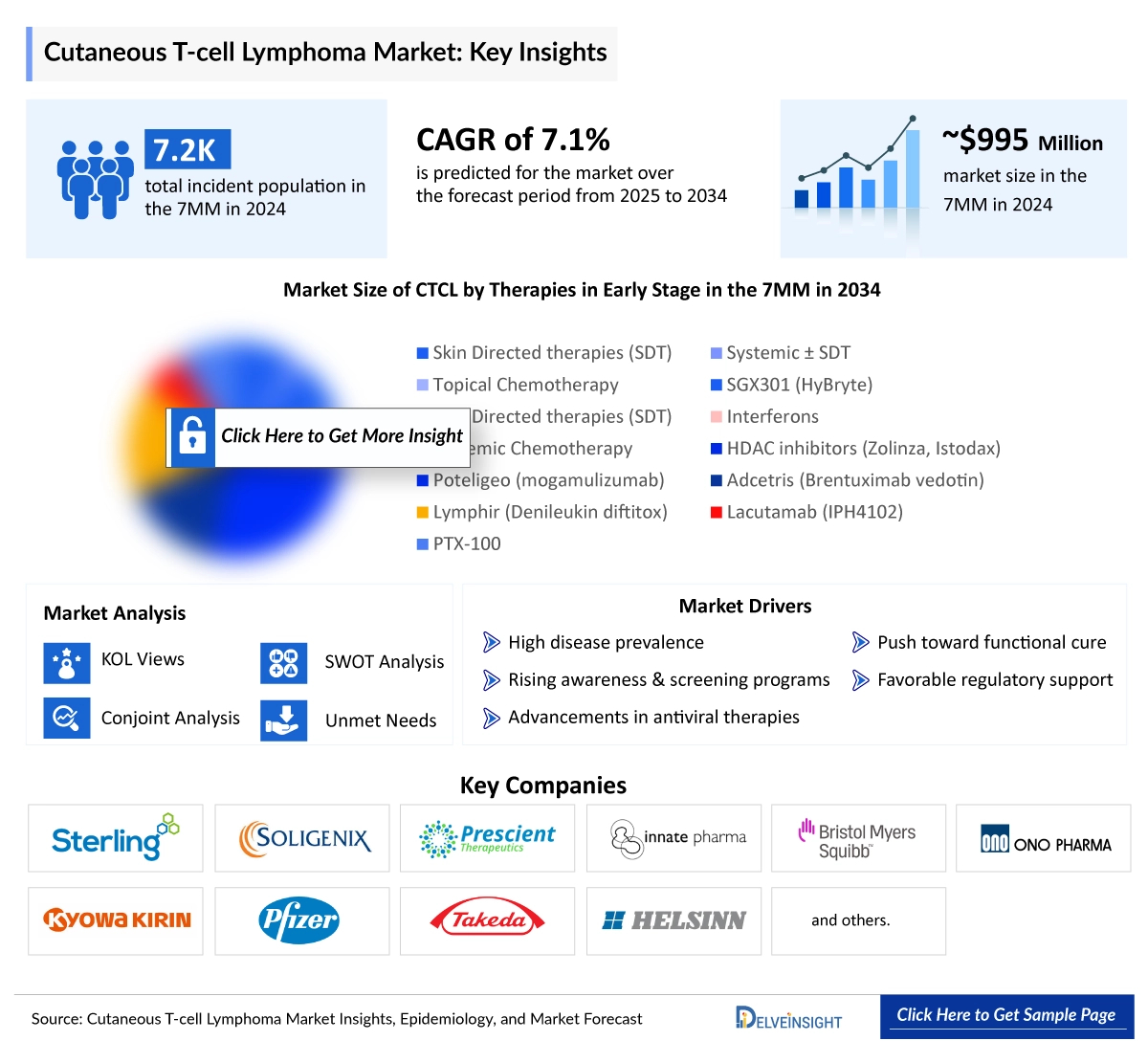
In 2025, the Cutaneous T-Cell Lymphoma (CTCL) market size across the 7MM was valued at approximately USD 1,042 million and is projected to expand at a CAGR of 5.0%, reaching around USD 1,622 million by 2034. Among these markets, the United States held the dominant position in 2024, capturing nearly 70% of the total CTCL market share within the 7MM. DelveInsight’s comprehensive market research provides critical insights into such market trends, enabling stakeholders to understand growth drivers, emerging opportunities, and potential challenges within the Cutaneous T-cell Lymphoma landscape.
In 2024, the United States reported the highest number of Cutaneous T-Cell Lymphoma (CTCL) cases among the 7MM, with approximately 3,050 cases, and this number is projected to rise by 2034. Across the 7MM, there were around 7,250 total incident CTCL cases in 2024, with a higher prevalence among males. In the U.S., mycosis fungoides represented the most common CTCL subtype. Within the EU4 and the UK, Germany recorded the highest number of cases, estimated at around 760 in 2024.
Approved therapies for Cutaneous T-cell Lymphoma (CTCL) encompass various mechanisms of action (MoA), including CCR4 inhibitors (POTELIGEO), CD30-targeted antibody-drug conjugates (ADCETRIS), HDAC inhibitors (ZOLINZA), and retinoids (TARGRETIN), providing a diverse range of treatment options.
KINSELBY (4SC), an investigational oral HDAC inhibitor, received a negative opinion from the Committee for Medicinal Products for Human Use (CHMP) regarding its Marketing Authorization Application (MAA), resulting in the discontinuation of its development and commercialization efforts. Cutaneous T-cell lymphomas (CTCLs) are a diverse group of extranodal non-Hodgkin lymphomas (NHL) arising from mature, skin-targeting T cells that primarily affect the skin. The predominant subtypes, mycosis fungoides and primary cutaneous CD30+ anaplastic large cell lymphoma (pcALCL), together account for approximately 80–85% of all CTCL cases.
DelveInsight’s report “Cutaneous T-cell Lymphoma Market Insights, Epidemiology, and Market Forecast-2034” provides a comprehensive analysis of the Cutaneous T-cell Lymphoma (CTCL) landscape. The report delivers detailed insights into the disease, including historical and projected epidemiology, helping stakeholders understand the prevalence, incidence, and patient demographics across key regions.
To Know in detail about the Cutaneous T-cell Lymphoma market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Cutaneous T-cell Lymphoma Market Forecast
Some of the key facts of the Cutaneous T-cell Lymphoma Market Report:
- Key Cutaneous T-cell Lymphoma Companies: Citius Pharmaceuticals, Soligenix, 4SC AG, Innate Pharma, Galderma R&D, Kyowa Kirin, Inc, oligenix, Ligand Pharmaceuticals, Bausch Health Americas, Inc., BioCryst Pharmaceuticals, Novartis, Celgene, Soligenix, Pfizer, Bioniz Therapeutics, Eisai Inc., Merck Sharp & Dohme LLC, and others
- Key Cutaneous T-cell Lymphoma Therapies: I/ONTAK, HyBryte, Resminostat, Lacutamab, CD11301, Mogamulizumab, Hypericin, ONTAK (denileukin difitox, DAB389IL-2), Bexarotene, Forodesine 200 mg, Panobinostat, romidepsin (depsipeptide, FK228), SGX301 (synthetic hypericin), Ritlecitinib, BNZ132-1-40, ONTAK Pembrolizumab, and others
- According to the research conducted by Rangoonwala et al. in 2022, Mycosis Fungoides (MF) stands as the most prevalent subtype among Cutaneous T-cell Lymphomas (CTCLs), accounting for roughly 50% of all CTCL cases. The incidence rate of MF appears to be nearly twice as frequent in males, with individuals typically diagnosed at a median age between 55 and 60 years old.
- The Cutaneous T-cell Lymphoma market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Cutaneous T-cell Lymphoma pipeline products will significantly revolutionize the Cutaneous T-cell Lymphoma market dynamics.
Cutaneous T-cell Lymphoma Overview
Cutaneous T-cell lymphoma (CTCL) is a rare type of non-Hodgkin lymphoma that primarily affects the skin. It arises when T-cells (a type of white blood cell) become cancerous and target the skin, leading to red, scaly patches, plaques, or tumors. The most common forms of CTCL include Mycosis Fungoides and Sézary Syndrome. While it often progresses slowly, advanced stages may affect lymph nodes, blood, and internal organs. Treatment depends on the disease stage and may involve topical therapies, phototherapy, systemic medications, or immunotherapy.
Get a Free sample for the Cutaneous T-cell Lymphoma Market Forecast, Size & Share Analysis Report:
https://www.delveinsight.com/report-store/cutaneous-t-cell-lymphoma-ctcl-market
Key Trends in Cutaneous T-cell Lymphoma Therapeutics Market:
- Advancements in Targeted Therapies: Growing use of targeted agents such as histone deacetylase (HDAC) inhibitors and monoclonal antibodies is improving treatment precision and outcomes in CTCL.
- Emergence of Immunotherapies: Checkpoint inhibitors and immune modulators are being actively explored to enhance immune system response against malignant T cells.
- Personalized Treatment Approaches: Increased focus on biomarker-driven therapies and genetic profiling is enabling tailored treatment strategies for better efficacy and reduced toxicity.
- Rising Clinical Research and Collaborations: Expanding clinical trials, partnerships, and R&D investments by pharmaceutical companies are accelerating innovation and market growth in CTCL therapeutics.
Cutaneous T-cell Lymphoma Epidemiology
The Cutaneous T-cell Lymphoma epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2020 to 2034. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Cutaneous T-cell Lymphoma Epidemiology Segmentation:
The Cutaneous T-cell Lymphoma market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Incident Cases of CTCL
- Type-specific Cases of CTCL
- Gender-specific Cases of CTCL
- Stage-specific Cases of CTCL
- Treatment-eligible pool in Early and Advanced Stages CTCL
Download the report to understand which factors are driving Cutaneous T-cell Lymphoma epidemiology trends @ Cutaneous T-cell Lymphoma Epidemiology Forecast
Recent Development In The Cutaneous T-cell Lymphoma Treatment Landscape:
- In October 2025, Soligenix, Inc. (Nasdaq: SNGX), a late-stage biopharmaceutical company dedicated to developing treatments for rare diseases with unmet medical needs, announced the completion of its first Data Monitoring Committee (DMC) review for the confirmatory Phase 3 trial evaluating HyBryte™ (synthetic hypericin) in Cutaneous T-cell Lymphoma (CTCL). The DMC found no safety issues, confirming that HyBryte™ maintains a favorable safety profile consistent with earlier clinical data. The ongoing Phase 3 FLASH2 (Fluorescent Light Activated Synthetic Hypericin 2) study builds upon the successful prior Phase 3 (FLASH) trial, a comparative study (HPN-CTCL-04), and an investigator-initiated study (RW-HPN-MF-01). With steady enrollment progress, Soligenix expects to provide an update in Q4 2025 and plans a pre-specified blinded interim efficacy analysis in the first half of 2026.
- In May 2025, Innate Pharma SA (Euronext Paris: IPH; Nasdaq: IPHA) announced that it will present long-term follow-up data from the Phase 2 TELLOMAK trial evaluating lacutamab, an anti-KIR3DL2 monoclonal antibody, in patients with Sézary syndrome (SS) and mycosis fungoides (MF)—two rare and aggressive forms of cutaneous T-cell lymphoma (CTCL). The results will be presented at the upcoming American Society of Clinical Oncology (ASCO) 2025 Annual Meeting in Chicago, Illinois.
- In April 2025, Interim findings from the ongoing FLASH2 study (NCT06470451) demonstrate that SGX301 (HyBryte) continues to show strong potential in treating early-stage cutaneous T-cell lymphoma (CTCL). Of the first nine enrolled patients, complete week 18 data were available for eight, and 75% achieved “treatment success” by week 18. Treatment success was defined as a ≥50% reduction from baseline in the cumulative modified Composite Assessment of Index Lesion Severity (mCAILS) score, with six out of eight evaluable patients meeting this benchmark, reflecting promising therapeutic outcomes.
- In February 2025, Innate Pharma has been granted Breakthrough Therapy Designation (BTD) by the U.S. Food and Drug Administration (FDA) for its lead candidate, lacutamab, aimed at treating adults with relapsed or refractory Sézary syndrome. Lacutamab is an innovative monoclonal antibody that selectively targets the killer cell immunoglobulin-like receptor 3DL2 (KIR3DL2) to induce cytotoxic responses. The therapy is currently being evaluated in clinical trials for its potential effectiveness in treating cutaneous T-cell lymphoma (CTCL) and peripheral T-cell lymphoma (PTCL).
- In January 2025, Interim findings from the investigator-initiated Phase 2 RW-HPN-MF-01 trial (NCT05872854), reported by Soligenix, revealed that treatment with synthetic hypericin (HyBryte) for up to 12 months produced positive clinical responses in patients with early-stage cutaneous T-cell lymphoma (CTCL).
- In January 2025, 4SC AG reported that it has successfully submitted its responses to the European Medicines Agency (EMA) Day-120 List of Questions in December 2024, as planned, following the initial Marketing Authorisation Application (MAA) for resminostat (Kinselby) filed in March 2024.
- In January 2025, Soligenix, Inc. (Nasdaq: SNGX), a late-stage biopharmaceutical company focused on developing therapies for rare diseases with high unmet needs, provided an interim update from an open-label, investigator-initiated study evaluating HyBryte™ (synthetic hypericin) for up to 12 months in patients with early-stage cutaneous T-cell lymphoma (CTCL). The study is led by Dr. Ellen Kim, Director of the Penn Cutaneous Lymphoma Program and Professor of Dermatology at the Hospital of the University of Pennsylvania, who also served as a lead investigator in the Phase 3 FLASH trial of HyBryte™ in early-stage CTCL.
- In December 2024, Soligenix, Inc. (Nasdaq: SNGX), a late-stage biopharmaceutical company focused on developing and commercializing therapies for rare diseases with high unmet needs, has announced the initiation of patient enrollment for its confirmatory Phase 3 clinical trial of HyBryte™ (synthetic hypericin) for the treatment of cutaneous T-cell lymphoma (CTCL). This new study, titled FLASH2 (Fluorescent Light Activated Synthetic Hypericin 2), is designed to validate findings from the prior statistically significant Phase 3 FLASH trial. It is further supported by data from a recent successful comparative study (HPN-CTCL-04) and an ongoing investigator-initiated trial, reinforcing the trial’s foundation.
Cutaneous T-cell Lymphoma Drugs Uptake and Pipeline Development Activities
The drugs uptake section focuses on the rate of uptake of the potential drugs recently launched in the Cutaneous T-cell Lymphoma market or expected to get launched during the study period. The analysis covers Cutaneous T-cell Lymphoma market uptake by drugs, patient uptake by therapies, and sales of each drug.
Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Cutaneous T-cell Lymphoma Pipeline Development Activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyzes recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Cutaneous T-cell Lymphoma Therapies and Key Companies
- I/ONTAK: Citius Pharmaceuticals
- HyBryte: Soligenix
- Resminostat: 4SC AG
- Lacutamab: Innate Pharma
- CD11301: Galderma R&D
- Mogamulizumab: Kyowa Kirin, In
- Hypericin: oligenix
- ONTAK (denileukin difitox, DAB389IL-2): Ligand Pharmaceuticals
- Bexarotene: Bausch Health Americas, Inc.
- Forodesine 200 mg: BioCryst Pharmaceuticals
- Panobinostat: Novartis
- romidepsin (depsipeptide, FK228): Celgene
- SGX301 (synthetic hypericin): Soligenix
- Ritlecitinib: Pfizer
- BNZ132-1-40: Bioniz Therapeutics
- ONTAK: Eisai Inc.
- Pembrolizumab: Merck Sharp & Dohme LLC
Discover more about therapies set to grab major Cutaneous T-cell Lymphoma market share @ Cutaneous T-cell Lymphoma Treatment Landscape
Cutaneous T-cell Lymphoma Market Drivers
- Rising Disease Awareness and Diagnosis: Improved understanding and early detection of CTCL are increasing the patient pool eligible for treatment.
- Advancements in Targeted and Immunotherapies: Development of novel therapies, such as monoclonal antibodies and checkpoint inhibitors, is enhancing treatment effectiveness and driving market growth.
- Growing R&D Investments: Increased focus by pharmaceutical companies on rare cancers like CTCL is expanding the therapeutic pipeline.
- Supportive Regulatory Approvals: Fast-track and orphan drug designations are encouraging innovation and accelerating product launches.
Cutaneous T-cell Lymphoma Market Barriers
- High Treatment Costs: Expensive targeted therapies limit patient accessibility, particularly in developing regions.
- Limited Therapeutic Options: Despite advances, there remains a lack of curative treatments, with many therapies offering only symptom control.
- Low Disease Prevalence: The rarity of CTCL poses challenges for large-scale clinical trials and investment returns.
- Adverse Effects and Resistance Issues: Long-term use of certain drugs can lead to side effects or resistance, impacting treatment adherence and outcomes.
Scope of the Cutaneous T-cell Lymphoma Market Report
- Study Period: 2020–2034
- Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
- Key Cutaneous T-cell Lymphoma Companies: Citius Pharmaceuticals, Soligenix, 4SC AG, Innate Pharma, Galderma R&D, Kyowa Kirin, Inc, oligenix, Ligand Pharmaceuticals, Bausch Health Americas, Inc., BioCryst Pharmaceuticals, Novartis, Celgene, Soligenix, Pfizer, Bioniz Therapeutics, Eisai Inc., Merck Sharp & Dohme LLC, and others
- Key Cutaneous T-cell Lymphoma Therapies: I/ONTAK, HyBryte, Resminostat, Lacutamab, CD11301, Mogamulizumab, Hypericin, ONTAK (denileukin difitox, DAB389IL-2), Bexarotene, Forodesine 200 mg, Panobinostat, romidepsin (depsipeptide, FK228), SGX301 (synthetic hypericin), Ritlecitinib, BNZ132-1-40, ONTAK Pembrolizumab, and others
- Cutaneous T-cell Lymphoma Therapeutic Assessment: Cutaneous T-cell Lymphoma current marketed and Cutaneous T-cell Lymphoma emerging therapies
- Cutaneous T-cell Lymphoma Market Dynamics: Cutaneous T-cell Lymphoma market drivers and Cutaneous T-cell Lymphoma market barriers
- Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies
- Cutaneous T-cell Lymphoma Unmet Needs, KOL’s views, Analyst’s views, Cutaneous T-cell Lymphoma Market Access and Reimbursement
To know more about Cutaneous T-cell Lymphoma companies working in the treatment market, visit @ Cutaneous T-cell Lymphoma Clinical Trials and Therapeutic Assessment
Need more?
- ✅ Connect with our analyst to learn how this research was developed
- ✅ Expand the scope with additional segments or countries through free customization
- ✅ Discover how this report can directly influence your business growth
Related Reports
Cutaneous T-Cell Lymphoma (CTCL) - Pipeline Insight, 2025
Cutaneous T-Cell Lymphoma (CTCL) Pipeline Insights, 2025 report by DelveInsight outlays comprehensive insights of present clinical development scenario and growth prospects across..
Cutaneous T-Cell Lymphoma(CTCL) - Epidemiology Forecast - 2034
DelveInsight's Cutaneous T-Cell Lymphoma(CTCL) - Epidemiology Forecast 2034 report delivers an in-depth understanding of the disease, historical, and forecasted epidemiology of Cutaneous
-pipeline.png&w=256&q=75)

