Achondroplasia Market
- The achondroplasia market size is projected to experience a rise in Compound Annual Growth Rate (CAGR) during the forecast period. Achondroplasia Market revenue growth is mainly due to advancements in genetic therapies, increased awareness and diagnosis rates, and a rise in research and development investments, which will lead to improved treatment options and better patient outcomes.
- DelveInsight estimates that the US accounted for nearly 14,500 diagnosed prevalent cases of achondroplasia in 2023.
- The dynamics of the Achondroplasia therapeutics market are anticipated to change in the coming years owing to the improvement in novel treatment options, which will increase healthcare spending worldwide. Key Achondroplasia companies in the treatment and prevention landscape are BioMarin Pharmaceuticals, Tyra Biosciences, Inc., RIBOMIC Inc., Bridge Bio Inc., Sanofi, and others.
- In November 2021, the US FDA approved the first treatment for Achondroplasia. The drug VOXZOGO (vosoritide) was approved through an accelerated approval pathway because it accomplished the disease's unmet medical need.
- Despite advancements in treatment options, several unmet needs persist in the management of Achondroplasia. These include the development of effective, targeted therapies, improved access to specialized care, enhanced early diagnosis, and comprehensive support systems to address the physical, emotional, and social challenges faced by patients and their families. DelveInsight’s comprehensive report titled “Achondroplasia Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Achondroplasia.
- The Achondroplasia market report presents historical and projected epidemiological data covering Total Diagnosed Prevalent Cases of Achondroplasia, Age-specific Diagnosed Prevalent Cases of Achondroplasia, and Gender-specific Diagnosed Prevalent Cases of Achondroplasia In addition to epidemiology, the Achondroplasia treatment market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the Achondroplasia treatment market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
- The Achondroplasia treatment market report analyzes the existing treatment practices and unmet medical requirements in Achondroplasia. It evaluates the Achondroplasia market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the Achondroplasia treatment market.
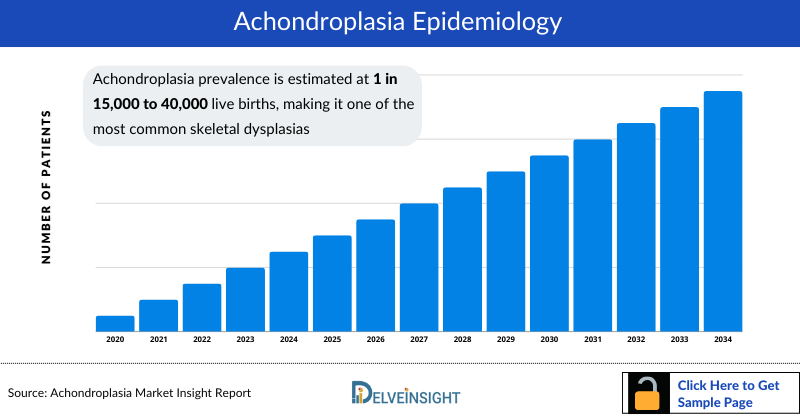
- Achondroplasia prevalence is estimated at 1 in 15,000 to 40,000 live births, making it one of the most common skeletal dysplasias.
Key Factors Driving Achondroplasia Market
Achondroplasia Patient Pool and Rising Prevalence
Achondroplasia, one of the most common skeletal dysplasias, has an estimated prevalence of 1 in 15K to 40K live births. According to DelveInsight’s estimates, the United States accounted for nearly 14.5K diagnosed prevalent cases in 2023, with numbers projected to rise by 2034 due to increased awareness, improved diagnostic techniques, and broader access to genetic testing. The expanding patient pool across the 7MM, including the US, EU4, the UK, and Japan, underscores growing clinical recognition and the need for effective, disease-modifying therapies.
Achondroplasia Market Growth and Treatment Advancements
The Achondroplasia market is projected to witness a notable rise in CAGR during the forecast period from 2025 to 2034, driven by advancements in genetic and targeted therapies, increased R&D investment, and improved diagnosis and treatment accessibility. Market revenue growth is further supported by the entry of first-in-class therapies that directly target the underlying FGFR3 pathway, offering improvements in growth velocity and quality of life. Despite progress, unmet needs remain, including limited long-term efficacy data, restricted access to specialized care, and the need for pediatric-to-adult management continuity.
Achondroplasia Marketed and Emerging Therapies
VOXZOGO (vosoritide) by BioMarin Pharmaceuticals is the first approved therapy for achondroplasia, enhancing bone growth by counteracting overactive FGFR3 signaling through daily subcutaneous injections. Emerging therapies under clinical investigation include RBM-007 (RIBOMIC Inc.), SAR-442501 (Sanofi), and low-dose infigratinib (BridgeBio Inc.), targeting FGFR3 or related pathways to promote skeletal growth and improve patient outcomes. These clinical trials are shaping a competitive landscape and expanding future treatment options.
Achondroplasia Clinical Trials and Competitive Landscape
Multiple Phase II and III trials are ongoing, evaluating the safety and efficacy of novel therapies such as RBM-007, SAR-442501, and infigratinib. BioMarin continues post-marketing studies for Voxzogo, while BridgeBio advances its PROPEL 3 Phase III trial. The competitive landscape includes BioMarin Pharmaceuticals, BridgeBio Inc., RIBOMIC Inc., Sanofi, Tyra Biosciences, and others, reflecting strong innovation in precision genetic therapies for achondroplasia.
Achondroplasia Treatment Market
Achondroplasia is a rare genetic bone growth disorder that results in marked short stature (dwarfism) due to a mutation in the fibroblast growth factor receptor 3 (FGFR3) gene. The mutation leads to a gain-of-function of the FGFR3 gene, which slows down the formation of bone in the cartilage of the growth plate and impairs growth in almost all bones in the body.
It is inherited in an autosomal dominant pattern, and the gene is fully penetrant. However, the condition occurs in over 80% of cases due to sporadic or de novo mutation. Thus, a child with achondroplasia can be born to healthy parents without a family history.
The disorder is characterized by distinctive features including short stature, a considerable head (macrocephaly) with a prominent forehead (frontal bossing) and flat (depressed) nasal bridge, short arms, and legs; prominent abdomen and buttocks (due to an inward curve of the spine), and short hands with fingers that assume a “trident” or three-pronged position during extension. Further, most females with achondroplasia naturally grow to approximately 4 feet 2 inches (128 cm), while males to about 4 feet 4 inches (134 cm).
Achondroplasia Diagnosis and Treatment Algorithm
Achondroplasia is typically diagnosed through clinical evaluation, genetic testing, and radiographic imaging. Clinicians assess physical characteristics such as disproportionate short stature, shortened limbs, and distinctive facial features. Prenatal ultrasound can often detect signs of Achondroplasia by the second trimester, identifying abnormalities in limb length and head size. Genetic testing confirms the diagnosis by identifying mutations in the FGFR3 gene, responsible for this condition. Postnatal diagnosis involves detailed physical examination and X-rays to observe characteristic skeletal abnormalities, such as shortening of the long bones, spinal stenosis, and specific features of the pelvis and skull.
While there is no cure for Achondroplasia, treatment focuses on managing symptoms and complications. Multidisciplinary care is essential, involving pediatricians, geneticists, orthopedists, neurologists, and physical therapists. Growth hormone therapy has shown some effectiveness in increasing height, though it is not a standard treatment. Surgical interventions may be necessary for severe skeletal abnormalities, such as limb lengthening procedures or surgeries to address spinal stenosis and other complications. Supportive therapies, including physical therapy and occupational therapy, help improve mobility and daily functioning. Additionally, new treatments targeting the underlying genetic mutation, such as C-type natriuretic peptide analogs, are under investigation and show promise in potentially altering disease progression. Regular monitoring and early intervention are crucial to optimize outcomes and improve the quality of life for individuals with Achondroplasia.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Achondroplasia Market |
|
|
Achondroplasia Market Size | |
|
Achondroplasia Companies |
Ascendis Pharma, QED Therapeutics (BridgeBio), Novartis, Sanofi, RIBOMIC, and others. |
|
Achondroplasia Epidemiology Segmentation |
|
Achondroplasia Epidemiology
The epidemiology section on the Achondroplasia epidemiology segment offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the Achondroplasia market report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the incidence of Achondroplasia. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- The total diagnosed prevalent cases of Achondroplasia in Japan and the US were ~18,000 in 2023, and they are expected to rise in the future.
- In the US, achondroplasia affects a slightly higher percentage of females (51.2%) compared to males (48.8%), possibly due to genetic or biological factors contributing to the marginally more significant occurrence among females.
- Japan had nearly 3000 total diagnosed prevalent cases of Achondroplasia in 2023.
- According to the epidemiology model, the age-specific cases are divided into nine groups: 0–4, 5–10, 11–15, 16–20, 21–30, 31–40, 41–50, 51–60, and >60. Based on DelveInsight’s estimates, the people in the age group 5–10 years of age were affected the most in the US, accounting for approximately 4000 cases in 2023.
- Achondroplasia prevalence is estimated at 1 in 15,000 to 40,000 live births, making it one of the most common skeletal dysplasias.
Achondroplasia Market Outlook
Achondroplasia, a common form of dwarfism caused by a genetic mutation, has historically been managed through supportive care and surgical interventions. However, recent advancements in medical science have introduced promising pharmacological treatments. Traditional management includes orthopedic surgeries to correct bone deformities, alleviate spinal stenosis, and enhance mobility. These procedures, though effective in addressing specific complications, do not alter the underlying growth deficiency.
The introduction of pharmacological treatments marks a significant shift in the management of achondroplasia. Vosoritide, a daily injectable drug, has emerged as a breakthrough therapy. Vosoritide is an analog of C-type natriuretic peptide (CNP) that targets the overactive fibroblast growth factor receptor 3 (FGFR3) pathway, which is responsible for inhibiting bone growth in individuals with achondroplasia. Achondroplasia Clinical trials have demonstrated that vosoritide significantly improves annual growth velocity in children with achondroplasia, offering a potential means to enhance final adult height and reduce complications associated with disproportionate growth.
Another promising treatment under investigation is transition CNP, a prodrug designed to provide sustained CNP release, which aims to reduce the frequency of dosing while maintaining efficacy. Early studies indicate that transCon CNP has the potential to improve growth outcomes and quality of life for patients with achondroplasia.
Beyond these pharmacological approaches, gene therapy is an area of active research. Scientists are exploring methods to correct the underlying FGFR3 mutation at the genetic level, potentially offering a one-time cure for achondroplasia. Although still in the experimental stages, gene therapy holds immense promise for transforming the future landscape of achondroplasia treatment market.
Overall, the evolution of treatment options for achondroplasia from purely supportive measures to innovative pharmacological and genetic therapies offers new hope for improving the lives of individuals with this condition. These advancements not only target the symptoms but also address the root cause of achondroplasia, paving the way for more effective and comprehensive management.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Achondroplasia therapeutics market in the 7MM is projected to grow significantly during the study period 2020–2034.
Achondroplasia Drug Chapters
Marketed Achondroplasia Drugs
VOXZOGO (vosoritide): BioMarin Pharmaceuticals
VOXZOGO (vosoritide) by BioMarin Pharmaceuticals is a groundbreaking treatment for achondroplasia. It works by mimicking C-type natriuretic peptide (CNP), which counteracts the overactive FGFR3 pathway that impairs bone growth. Administered via daily subcutaneous injections, Voxzogo enhances growth velocity in children with achondroplasia. It received FDA approval in November 2021 and EMA approval in August 2021, making it the first pharmacological treatment approved explicitly for this genetic condition.
Emerging Achondroplasia Drugs
RBM-007: RIBOMIC Inc.
RBM-007, developed by RIBOMIC Inc., is an RNA aptamer targeting fibroblast growth factor 2 (FGF2) for the treatment of achondroplasia. Its mechanism of action involves inhibiting FGF2, thereby promoting bone growth and reducing the effects of the FGFR3 mutation responsible for achondroplasia. RBM-007 is administered via subcutaneous injection. It is currently in Phase IIa achondroplasia clinical trials, assessing its safety and preliminary efficacy in affected individuals.
SAR-442501: Sanofi
SAR-442501, developed by Sanofi, is a novel treatment for achondroplasia. It functions as a selective antagonist of the fibroblast growth factor receptor 3 (FGFR3), which is implicated in inhibiting bone growth in achondroplasia. By blocking this receptor, SAR-442501 aims to enhance skeletal growth and improve height outcomes. The drug is administered via subcutaneous injection. The upreACH Phase II study of SAR442501 in pediatric patients with achondroplasia will remain ongoing but is not expected to advance further.
Low dose infigratinib: BridgeBio Inc.
Infigratinib is an oral small molecule in development for the treatment of FGFR-driven conditions, including achondroplasia, a bone growth disorder in children. Over-activating FGFR3 mutations drive downstream MAPK and STAT1 signaling that aberrates growth plate development, thereby causing disproportionate short stature and severe health complications stemming from cranial and spinal issues. Low-dose infigratinib has the potential to help children due to its direct inhibition of the mutant FGFR3 receptor. BridgeBio initiated PROPEL 3, a Phase III clinical trial studying the efficacy and safety of infigratinib in children with achondroplasia. Both the US FDA and the EU EMA indicated the trial design for PROPEL 3 would be acceptable as a registrational study to support a marketing application for the treatment of children with achondroplasia.
|
MoA |
RoA |
Company |
Logo |
|
Fibroblast growth factor inhibitors |
SC |
RIBOMIC Inc. |
|
|
Type 3 fibroblast growth factor receptor modulators |
SC |
Sanofi |
|
|
XX |
XX |
XX |
|
Note: Detailed emerging therapies assessment will be provided in the final report.
Achondroplasia Market Segmentation
DelveInsight’s Achondroplasia Market Insights, Epidemiology, and Market Forecast—2034 report provides a detailed outlook of the current and future Achondroplasia treatment market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future Achondroplasia market share of all therapies.
Achondroplasia Market Size by Countries
The Achondroplasia market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Achondroplasia treatment market, primarily attributed to the elevated cost of the available treatments and the increasing sensitivity toward pneumococcal infections. This dominance is projected to persist, especially with the potential early introduction of new Achondroplasia products.
Achondroplasia Market Size by Therapies
Achondroplasia Market Size by Therapies is categorized into current and emerging Achondroplasia treatment market for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is low-dose infigratinib, which is in BridgeBio Inc.'s developmental pipeline.
Note: Detailed market segment assessment will be provided in the final report.
Achondroplasia Drugs Uptake
The Achondroplasia drugs market is growing due to rising prevalence, with increasing demand for treatments targeting the genetic disorder's symptoms. This section focuses on the sales uptake of potential Achondroplasia drugs that have recently been launched or are anticipated to be launched in the Achondroplasia market between 2020 and 2034. It estimates the market penetration of Achondroplasia drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Achondroplasia market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Achondroplasia
Achondroplasia Market Access and Reimbursement
DelveInsight’s ‘Achondroplasia Market Insights, Epidemiology, and Market Forecast—2034’ report provides a descriptive overview of the Achondroplasia market access and reimbursement scenario.
This section includes a detailed analysis of each therapy's country-wise healthcare system, enlightening market access, reimbursement policies, and health technology assessments.
KOL Views on Achondroplasia
To keep up with current Achondroplasia market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Achondroplasia domain. Their opinion helps understand and validate current and emerging Achondroplasia therapies and treatment patterns for Achondroplasia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market of Achondroplasia unmet needs.
Achondroplasia: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as Greenberg Center for Skeletal Dysplasias, Department of Genetic Medicine, Johns Hopkins University, Baltimore, USA; McGovern Medical School, University of Texas Health, Houston, TX, USA; Department of Pediatrics, IRCCS Istituto Giannina Gaslini, Genoa, Italy; Instituto de Investigación Biomédica de Málaga-IBIMA, Hospital Universitario Virgen de la Victoria, Málaga, Spain; Department of Medical Genetics, Tokyo Medical University, Tokyo Japan; and others.
“Achondroplasia occurs in approximately 1 in 25,000 births globally. Although treatments focus on managing symptoms, there is a need for research into therapies that address the genetic basis of the condition.”
Note: Detailed assessment of KOL Views will be provided in the full report on Achondroplasia.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Achondroplasia therapeutics market, utilizing various Competitive Intelligence tools such as SWOT analysis, Conjoint Analysis, and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
The emerging Achondroplasia therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Achondroplasia market.
Achondroplasia Pipeline Development Activities
The Achondroplasia treatment market report offers an analysis of different Achondroplasia clincial trials within Phase II and III stages and examines companies involved in developing targeted therapeutics for Achondroplasia. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Achondroplasia Clinical Trials Activities
The Achondroplasia treatment market report covers information on collaborations, acquisitions and mergers, licensing, patent details, and other aspects of emerging Achondroplasia therapies.
Achondroplasia Market Report Insights
- Achondroplasia Patient Population
- Therapeutic Approaches
- Achondroplasia Pipeline Analysis
- Achondroplasia Market Size
- Achondroplasia Market Trends
- Achondroplasia Market Opportunities
- Impact of Upcoming Achondroplasia Therapies
Achondroplasia Market Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Achondroplasia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Achondroplasia Market
- Achondroplasia Drugs Uptake
Achondroplasia Market Report Assessment
- Achondroplasia Current Treatment Practices
- Achondroplasia Unmet Needs
- Achondroplasia Pipeline Product Profiles
- Achondroplasia Market Attractiveness
- Achondroplasia Market Drivers
- Achondroplasia Market Barriers
Key Questions
- How common is Achondroplasia?
- What are the key findings of Achondroplasia epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Achondroplasia?
- What are the disease risk, burden, and unmet needs of Achondroplasia?
- At what CAGR is the Achondroplasia market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Achondroplasia market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Achondroplasia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Which country will have the highest number of Achondroplasia patients among EU4 and the UK during the forecast period (2020–2034)?
- How many Achondroplasia companies are currently developing therapies for the treatment of Achondroplasia?
Related Insights