Acute Respiratory Distress Syndrome Market
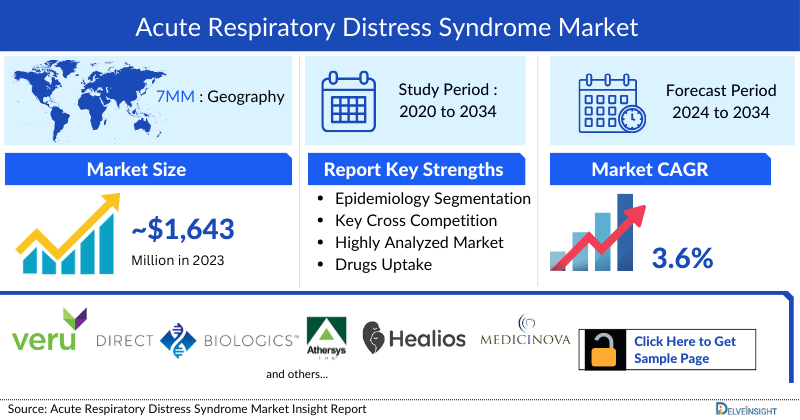
- In 2023, the Acute Respiratory Distress Syndrome Market Size was highest in the US among the 7MM accounting for approximately USD 1,643 million that is further expected to increase at a CAGR of 3.6%.
- Acute Respiratory Distress Syndrome is a critical condition characterized by the rapid onset of respiratory failure due to extensive inflammation in the lungs. This syndrome can arise from various acute injuries, leading to significant morbidity and mortality rates among affected individuals.
- The current Acute Respiratory Distress Syndrome Treatment Strategies mainly rely upon the use of Neuromuscular Blockades, Inhaled vasodilators, Corticosteroids, and others. Inhaled vasodilators accounted for highest market share of ~ 1,063 million in 2023, for treatment of Acute Respiratory Distress Syndrome in the 7MM.
- In August 2024, MediciNova received a Notice of Allowance from the US Patent and Trademark Office for a pending patent application related to MN-166 (ibudilast) for post-COVID conditions.
- The total Acute Respiratory Distress Syndrome Market is anticipated to experience growth during the forecast period due to expected entry of emerging therapies that includes Sabizabulin (VERU-111), ExoFlo, Invimestrocel, and Ibudilast.
- The Acute Respiratory Distress Syndrome Market is experiencing significant growth, driven by rising incidences of respiratory diseases, advancements in therapeutic options, and increased healthcare investments. The development of targeted treatments, including novel biologics and mechanical ventilation technologies, is further boosting the market demand.
Request for Unlocking the Sample Page @ Acute Respiratory Distress Syndrome Treatment Market
DelveInsight’s “Acute Respiratory Distress Syndrome Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Acute Respiratory Distress Syndrome, historical and forecasted epidemiology as well as the Acute Respiratory Distress Syndrome market trends in the United States, EU4, and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Acute Respiratory Distress Syndrome Treatment Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Acute Respiratory Distress Syndrome market size from 2020 to 2034. The Report also covers current Acute Respiratory Distress Syndrome treatment market practices, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Acute Respiratory Distress Syndrome Drugs Market |
|
|
Acute Respiratory Distress Syndrome Treatment Market Size | |
|
Acute Respiratory Distress Syndrome Companies |
|
|
Acute Respiratory Distress Syndrome Epidemiology |
|
Key Factors Driving the Acute Respiratory Distress Syndrome Market
Rising ARDS Incidence
The rise in the incidence of ARDS stimulates the research and development of the drug, as it is likely to provide an appropriate environment for newer products to be profitable. According to DelveInsight’s estimates, the total incident population of ARDS in the 7MM was estimated to be approximately 875K cases in 2024. These cases are projected to increase during the forecast period (2025-2034).
Dominance of Inhaled Vasodilators
The current acute respiratory distress syndrome treatment strategies mainly rely upon the use of Neuromuscular Blockades, inhaled vasodilators, corticosteroids, and others. Inhaled vasodilators accounted for the highest ARDS market share of ~USD 1 billion in 2023, for the treatment of acute respiratory distress syndrome in the 7MM.
Growing ARDS Clinical Trial Activity
Several ARDS therapies in clinical trials include MultiStem (HLCM051) by Healios Pharma, ExoFlo (DB-2Q) by Direct Biologics, Gelsolin by BioAegis Therapeutics, EB05 (paridiprubart) by Edesa Biotech and Light Chain Bio, and TP508 by Chrysalis BioTherapeutics, among others.
Acute Respiratory Distress Syndrome Treatment Market
ARDS is categorized into three severity levels based on the PaO2/FiO2 ratio, which helps guide treatment decisions: mild (200–300 mmHg), moderate (100–200 mmHg), and severe (<100 mmHg). ARDS may develop over a few days, or it can get worse very quickly. Usually, the first symptom of ARDS is shortness of breath. Besides, other signs and symptoms of ARDS are low blood oxygen, rapid breathing, and clicking, bubbling, or rattling sounds in the lungs when breathing.
Patients with ARDS typically present with severe shortness of breath, rapid breathing, and low blood oxygen levels, often accompanied by coughing and fatigue. The syndrome is frequently triggered by conditions such as pneumonia, sepsis, trauma, aspiration, and toxic inhalation. Various risk factors increase susceptibility to ARDS, including older age, chronic lung diseases like ARDS, obesity, and states of immunosuppression, which further complicate management and recovery. The pathophysiology of ARDS involves damage to the alveolar-capillary membrane, resulting in increased permeability and fluid accumulation within the alveoli. This leads to atelectasis, decreased lung compliance, and significant hypoxemia, while the inflammatory response contributes to ongoing lung injury.
Acute Respiratory Distress Syndrome Diagnosis
The diagnosis of Acute Respiratory Distress Syndrome is primarily based on clinical criteria and imaging studies. It begins with identifying symptoms such as severe shortness of breath, rapid breathing, and hypoxia (low blood oxygen levels), often following a known trigger like pneumonia, trauma, or sepsis. Chest X-rays or CT scans reveal bilateral infiltrates, which help exclude other conditions, such as heart failure.
The Berlin Definition (2012) is commonly used, categorizing ARDS based on the severity of hypoxemia—mild, moderate, or severe—assessed through the PaO2/FiO2 ratio. Additionally, arterial blood gas analysis is critical in confirming hypoxemia and ruling out other causes. Exclusion of cardiac causes of pulmonary edema is necessary to confirm ARDS, often achieved by evaluating left atrial pressure or conducting an echocardiogram. Early and accurate diagnosis is essential for timely intervention and improving patient outcomes.
Further details related to diagnosis are provided in the report…
Acute Respiratory Distress Syndrome Treatment
The treatment of Acute Respiratory Distress Syndrome focuses on supporting respiratory function and addressing the underlying cause. The primary management strategy involves mechanical ventilation with lung-protective strategies, such as low tidal volume ventilation (6 mL/kg of predicted body weight) and the use of positive end-expiratory pressure (PEEP) to prevent alveolar collapse. Prone positioning may also be utilized to improve oxygenation in severe cases. In some instances, extracorporeal membrane oxygenation (ECMO) is considered for patients who fail to respond to conventional ventilation.
Pharmacologically, corticosteroids are sometimes used to reduce inflammation, though their use remains controversial. Antibiotics and antivirals may be administered if infection is the underlying cause. Supportive care includes fluid management to avoid both under- and over-resuscitation, as well as careful monitoring of organ function. The treatment approach is tailored to the severity of ARDS and the patient’s overall condition, with multidisciplinary care often required for optimal outcomes.
Further details related to treatment are provided in the report…
Acute Respiratory Distress Syndrome Epidemiology
As the market is derived using a patient-based model, the Acute Respiratory Distress Syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Acute Respiratory Distress Syndrome, Severity-specific Incident Cases of Acute Respiratory Distress Syndrome, and Incident Cases of Acute Respiratory Distress Syndrome by Risk Factors, in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
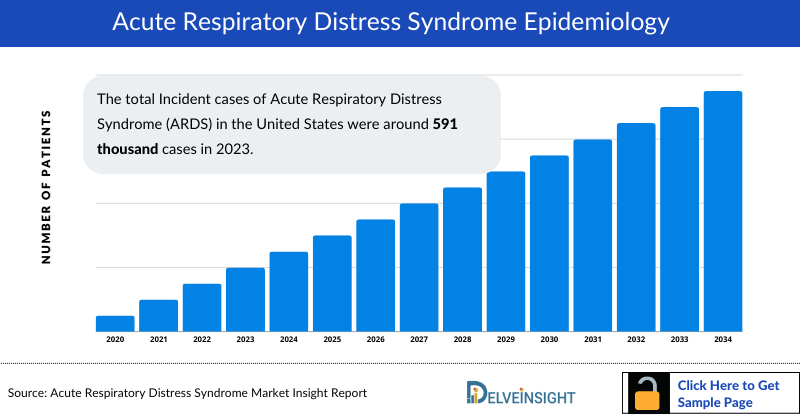
- The total Acute Respiratory Distress Syndrome incidence in the United States were around 591 thousand cases in 2023.
- The United States contributed to the largest incident population of Acute Respiratory Distress Syndrome, acquiring ~62% of the 7MM in 2023. Whereas, EU4 and the UK, and Japan accounted for around 34% and 4% of the total population share, respectively, in 2023.
- Among the EU4 countries, Germany accounted for the largest Acute Respiratory Distress Syndrome incidence (181 thousand Cases) cases followed by France (47 thousand Cases), whereas Spain accounted for the lowest number of cases (25 thousand Cases) in 2023.
- In 2023, it was estimated that there were around 177 thousand Incident cases were of Mild severity, 276 thousand cases of Moderate severity, and 138 thousand cases of Severe severity in the US.
- According to DelveInsight estimates, in 2023, among the risk factor-associated cases of ARDS in the US, there are approximately 210,560 incident cases of pneumonia, 175,076 cases of sepsis, 18,169 cases of trauma, 61,157 cases of aspiration, 8,974 cases of pancreatitis, 4,757 cases of COVID-19, 63,242 cases of other types, and about 49,340 cases of unknown types.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Acute Respiratory Distress Syndrome Prevalence
Acute Respiratory Distress Syndrome Drug Chapters
The drug chapter segment of the Acute Respiratory Distress Syndrome treatment market report encloses a detailed analysis of Acute Respiratory Distress Syndrome off-label drugs and late-stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Acute Respiratory Distress Syndrome clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Acute Respiratory Distress Syndrome news and press releases.
Acute Respiratory Distress Syndrome Emerging Drugs
- Sabizabulin (Veru-111): Veru
Sabizabulin (VERU-111) is an orally bioavailable bis-indole that binds to the colchicine binding site of alpha and beta tubulin and inhibits tubulin polymerization at low nanomolar concentrations. The drug disrupts the microtubules, the central mechanism that contributes to both their antiviral and anti-inflammatory activities, by disrupting the intracellular transport of viruses, such as SARS CoV-2, along microtubules. Microtubule trafficking is critical for viruses to cause infection.
In addition to having potent anti-inflammatory effects, microtubule depolymerization drugs that target the alpha and beta tubulin subunits of microtubules may be able to treat the cytokine release syndrome (cytokine storm) brought on by the SARS-CoV-2 viral infection, which appears to be linked to high COVID-19 mortality rates. The two-pronged therapy provided by sabizabulin for COVID-19 viral infection and the incapacitating and occasionally fatal respiratory consequences of the virus. First, it would directly influence S protein-microtubule trafficking as an antiviral, which might diminish the formation of infectious virions by influencing viral replication, assembly, and particle egress. Second, as an anti-inflammatory drug, it may lessen virally produced acute respiratory system inflammation and lower the frequency of cytokine storm and septic shock that leads to ARDS.
The company is currently investigating a Phase III study of VERU-111 for the treatment of SARS-CoV-2 in patients at high risk for ARDS. However, a planned interim analysis was conducted in the first 150 patients randomized into the study. In January 2022, the US FDA granted Fast Track Designation (FTD) for Sabizabulin for the treatment of hospitalized COVID-19 patients at high risk for ARDS.
In September 2023, Veru Inc. reported an agreement with the FDA on the design of a new Phase III clinical trial for sabizabulin in combination with standard care for patients with virus-induced ARDS. The randomized, placebo-controlled study will assess the efficacy and safety of a 9 mg oral daily dose of sabizabulin in hospitalized adult patients. This trial may serve as the sole study required for NDA submission. Additionally The Phase III (904) study involving hospitalized patients with viral ARDS is currently paused, according to the company’s latest pipeline update. The continuation of this study is contingent upon securing funding from government grants, partnerships with pharmaceutical companies, or similar third-party external sources. This indicates a dependency on external financial support to advance the study’s progress.
Further detail in the report…
- ExoFlo (DB-001): Direct Biologics
ExoFlo (DB-001) by Direct Biologics is an extracellular signal product isolated from human Bone Marrow Mesenchymal Stem or Stromal Cells (BM-MSCs) that contains growth factors and extracellular vesicles, including exosomes. ExoFlo provides natural bioactive signals that downregulate inflammation, direct cellular communication, and upregulate tissue repair in humans. These vesicles are 30–150 nm in size and are purified using proprietary cGMP processing. Recently, in July 2022, the company initiated the evaluation of the drug in Phase III for COVID-19 moderate-to-severe ARDS. In April 2022, the US FDA awarded ExoFlo a Regenerative Medicine Advanced Therapy (RMAT) designation for the treatment of ARDS associated with COVID-19.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Sabizabulin |
Inhibits tubulin polymerization |
Oral |
Veru Inc. |
III |
|
ExoFlo |
Inflammation modulator |
IV |
Direct Biologics |
III |
|
XXX |
XXX |
XXX |
XXX |
II |
Acute Respiratory Distress Syndrome Market Outlook
ARDS, a life-threatening condition, was described as a form of respiratory failure that closely resembled respiratory distress syndrome in infants. This can be caused by a variety of pulmonary (such as pneumonia and aspiration) or non-pulmonary (like sepsis, pancreatitis, and trauma) abuses, leading to the development of non-hydrostatic pulmonary edema. ARDS is characterized by an acute, diffuse, inflammatory lung injury, leading to increased alveolar-capillary permeability, increased lung weight, and loss of aerated lung tissue. Clinically, this establishes hypoxemia, with bilateral opacities on chest radiography, associated with reduced lung compliance and increased venous admixture and physiological dead space. Morphologically, diffuse alveolar damage is observed in the acute phase of ARDS.
Despite decades of research, treatment options for ARDS are restricted. Supportive care with mechanical ventilation remains the mainstay of management. There are relatively few treatments available for ARDS. Other treatment options to which the patients with ARDS are generally subjected include supplemental oxygen, prone positioning, use of paralytics, fluid management, and a technique called Positive End-expiratory Pressure (PEEP) to help push the fluid out of air sacs. These are combined with continuing treatment of the original illness or injury. Because people with ARDS are less able to fight lung infections, they may develop bacterial pneumonia during the illness. Antibiotics are given to fight infection. Also, supportive treatment, such as intravenous fluid or food, may be needed.
Alveolar flooding and pulmonary edema formation are important pathophysiological derangements in patients with ARDS. Experimental data have shown that ß2 agonists can increase sodium transport by activating ß2 receptors on alveolar type I and type II cells, accelerating the resolution of pulmonary edema. Because injury to the alveolar epithelium is a significant cause of ARDS, the acceleration of alveolar epithelial repair may assist in the resolution of pulmonary edema and lung injury. Keratinocyte Growth Factor (KGF) is important in alveolar epithelial repair, and experimental and human studies support the concept that KGF may be beneficial in patients with ARDS.
Inflammation is an additional pathological hallmark of ARDS and may contribute to both pulmonary and non-pulmonary organ failure. Statins can decrease inflammation and the development of lung injury in experimental models. Statins are a class of drugs that doctors often prescribe to help decrease cholesterol levels in the blood. By lowering the levels, heart attacks and stroke can be prevented. Several studies show that, in certain people, statins reduce the risk of heart attack, stroke, and even death from heart disease. However, some studies contradict STATINS use in the management of ARDS because statins neither provide benefits for lowering the morbidity of ARDS in high-risk patients nor improve the clinical outcomes of ALI/ARDS patients. Hence, it may not be appropriate to advocate statin use for the prevention and treatment of ALI/ARDS.
Statins are expected to reduce inflammation in the lungs of patients with ARDS due to their anti-inflammatory effects. Many clinical investigations have revealed contradictory results regarding the role of statin therapy in ARDS. Despite promising pathophysiological rationale and encouraging preclinical data, treatments like ß2 agonists, Keratinocyte Growth Factor (KGF), and statins are not currently recommended for the routine management of ARDS. While preclinical studies and some clinical trials suggest that ß2 agonists may help reduce pulmonary edema in acute lung injury and ARDS, their potential benefits have not yet been confirmed on a larger scale.
Given the lack of proven effectiveness for these treatments, management of ARDS typically involves alternative therapies such as neuromuscular blocking agents, inhaled vasodilators, and corticosteroids. These options have shown more established benefits, including improved oxygenation and reduced inflammation, making them part of the standard care for ARDS patients. Mechanical ventilation is the foundation of ARDS management. The use of protective ventilation is a priority in this acute phase of lung inflammation. According to various studies, Neuromuscular Blocking Agents (NMBAs) play an important role in the management of a large number of hospital patients. NMBAs induce reversible muscle paralysis. Their use in patients with ARDS remains controversial but occurs frequently. In addition to their routine use in surgical anesthesia, NMBAs may be valuable in many new and evolving critical care situations. NMBAs exert their pharmacologic effects by modulating signal transmission in skeletal muscle. Action potentials reaching skeletal muscle activate the release of acetylcholine into the motor endplates.
One of the primary clinical concerns for patients who develop ARDS is to reduce pressure and stress on the lungs, thereby reducing additional inflammation beyond the initial damage or insult. Because of their effects on diaphragmatic tone, NMBAs have been suggested as a method for decreasing ventilator asynchrony and pulmonary pressures. Three primary studies have been published that evaluated the use of NMBAs in the early phase of ARDS. Based on the findings of these studies, it is reasonable to consider NMBAs for the treatment of acute ARDS in patients presenting to the ICU.
Few new agents are being developed and tested as potential treatments for Acute Respiratory Distress Syndrome; the emerging drugs include Sabizabulin by Veru Inc., ExoFlo by Direct Biologics, MultiStem by Athersys/Healios, Ibudilast by MediciNova, and others.
- The Acute Respiratory Distress Syndrome Therapeutics Market Size in the 7MM was approximately USD 1,643 million in 2023.
- The Acute Respiratory Distress Syndrome Market Size in the 7MM will increase at a CAGR of 3.6% due to increasing awareness of the disease, better diagnosis, and the launch of the emerging therapy.
- The United States accounted for the highest Acute Respiratory Distress Syndrome Therapeutics Market Size approximately 74% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the European countries, Germany had the highest Acute Respiratory Distress Syndrome Market Size with nearly USD 206 million in 2023, while Spain had the lowest Acute Respiratory Distress Syndrome Market Size with ~USD 29 million in 2023.
- The Acute Respiratory Distress Syndrome Treatment Market Size in Japan was estimated to be ~USD 46 million in 2023, which accounts for 3% of the total 7MM market.
- With the expected launch of upcoming therapies, such as Sabizabulin the total Acute Respiratory Distress Syndrome Treatment Market Size is expected to show change in the upcoming years.
Recent Developments in the Acute Respiratory Distress Syndrome Market:
- In Sept 2025, Sentec received FDA 510(k) clearance for its LuMon Electrical Impedance Tomography (EIT) system, the first EIT technology approved in the U.S. for premature infants and spontaneously breathing patients. The system is especially beneficial for monitoring NICU patients at the bedside.
- On December 4, 2024, PIF Partners announced that its proprietary investigational therapeutic, 101-PGC-005 (‘005), for the treatment of systemic juvenile idiopathic arthritis (sJIA) flares, received Rare Pediatric Disease Designation from the FDA. The treatment, a Type IA prodrug of dexamethasone targeting CD206+ macrophages, is also in Phase 3 clinical trials in India for treating acute respiratory distress syndrome induced by COVID-19.
Acute Respiratory Distress Syndrome Drugs Uptake
This section focuses on the uptake rate of potential Acute Respiratory Distress Syndrome drugs expected to launch in the market during 2020–2034. For example, Sabizabulin in the US is expected to be launched by 2028 with a peak share of 12%. Sabizabulin is anticipated to take 7 years to peak with a medium uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Acute Respiratory Distress Syndrome Pipeline Development Activities
The Acute Respiratory Distress Syndrome therapeutics market report provides insights into different Acute Respiratory Distress Syndrome clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Acute Respiratory Distress Syndrome Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Acute Respiratory Distress Syndrome therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Acute Respiratory Distress Syndrome emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Acute Respiratory Distress Syndrome Treatment Drugs
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Acute Respiratory Distress Syndrome evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake` along with challenges related to accessibility, including KOL from Cardiovascular Research Institute, University of California San Francisco; University of Utah, Salt Lake City, US; Respiratory Institute, Cleveland, Ohio, US; Stanford University School of Medicine, US; ARDS and ECMO Centre, Witten/Herdecke University Hospital, Germany; Hôpital Raymond Poincaré (APHP), Garches, France; University of Angers, Angers, Italy; Hospital Universitario Rio Hortega, Valladolid, Spain; Hirosaki University Graduate School of Medicine, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Acute Respiratory Distress Syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Acute Respiratory Distress Syndrome Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Acute Respiratory Distress Syndrome treatment market landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Acute Respiratory Distress Syndrome Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Acute Respiratory Distress Syndrome therapeutics market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Acute Respiratory Distress Syndrome Market Report Scope
- The Acute Respiratory Distress Syndrome therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Acute Respiratory Distress Syndrome treatment market, historical and forecasted Acute Respiratory Distress Syndrome market size, Acute Respiratory Distress Syndrome drugs market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Acute Respiratory Distress Syndrome therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Acute Respiratory Distress Syndrome drugs market.
Acute Respiratory Distress Syndrome Market Report Insights
- Patient-based Acute Respiratory Distress Syndrome Market Forecasting
- Acute Respiratory Distress Syndrome Therapeutic Approaches
- Acute Respiratory Distress Syndrome Pipeline Drugs Analysis
- Acute Respiratory Distress Syndrome Market Size and Trends
- Existing and Future Acute Respiratory Distress Syndrome Drugs Market Opportunities
Acute Respiratory Distress Syndrome Market Report Key Strengths
- 11 years Acute Respiratory Distress Syndrome Market Forecast
- The 7MM Coverage
- Acute Respiratory Distress Syndrome Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Acute Respiratory Distress Syndrome Drugs Uptake
- Key Acute Respiratory Distress Syndrome Market Forecast Assumptions
Acute Respiratory Distress Syndrome Market Report Assessment
- Current Acute Respiratory Distress Syndrome Treatment Market Practices
- Acute Respiratory Distress Syndrome Unmet Needs
- Acute Respiratory Distress Syndrome Pipeline Drugs Profiles
- Acute Respiratory Distress Syndrome Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Acute Respiratory Distress Syndrome Market Drivers
- Acute Respiratory Distress Syndrome Market Barriers
Key Questions:
Acute Respiratory Distress Syndrome Treatment Market Insights:
- What was the Acute Respiratory Distress Syndrome drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Acute Respiratory Distress Syndrome market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Acute Respiratory Distress Syndrome market size during the forecast period (2024–2034)?
- At what CAGR, the Acute Respiratory Distress Syndrome market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Acute Respiratory Distress Syndrome market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Acute Respiratory Distress Syndrome market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Acute Respiratory Distress Syndrome Epidemiology Insights:
- What are the disease risk, burden, and Acute Respiratory Distress Syndrome Unmet Needs?
- What is the historical Acute Respiratory Distress Syndrome patient population in the United States, EU4 (Germany, France, Italy, Spain) and the UK, and Japan?
- What would be the forecasted patient population of Acute Respiratory Distress Syndrome at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Acute Respiratory Distress Syndrome?
- Out of the above-mentioned countries, which country would have the highest Incident population of Acute Respiratory Distress Syndrome during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Acute Respiratory Distress Syndrome Treatment Market Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the treatment of Acute Respiratory Distress Syndrome along with the approved therapy?
- What are the current treatment guidelines for the treatment of Acute Respiratory Distress Syndrome in the US, Europe, And Japan?
- What are the Acute Respiratory Distress Syndrome-marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many companies are developing therapies for the treatment of Acute Respiratory Distress Syndrome?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Acute Respiratory Distress Syndrome?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to the Acute Respiratory Distress Syndrome therapies?
- What are the recent therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for Acute Respiratory Distress Syndrome and their status?
- What are the key designations that have been granted for the emerging therapies for Acute Respiratory Distress Syndrome?
- What are the 7MM historical and forecasted market of Acute Respiratory Distress Syndrome?
Reasons to Buy
- The Acute Respiratory Distress Syndrome therapeutics market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Acute Respiratory Distress Syndrome Drugs Market.
- Insights on patient burden/disease Acute Respiratory Distress Syndrome Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Acute Respiratory Distress Syndrome drugs market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Acute Respiratory Distress Syndrome drugs market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles:
-market.png&w=256&q=75)


.png&w=3840&q=75)
