Adrenomyeloneuropathy (AMN) Market
- Adrenoleukodystrophy (ALD), also called X-linked Adrenoleukodystrophy (X-ALD), is a rare genetic disorder characterized by the impaired metabolism of very long-chain fatty acids (VLCFAs), leading to their accumulation in various tissues, especially the nervous system and adrenal glands.
- Adrenomyeloneuropathy is the most prevalent form of ALD, primarily affecting adult men. It typically begins with a gradual onset of leg stiffness and weakness, difficulty in walking, and pain or discomfort due to nerve damage. Additional symptoms may include bladder and bowel dysfunction, as well as sexual dysfunction. Adrenomyeloneuropathy is caused by genetic mutations in the ABCD1 gene. The ABCD1 gene provides the instructions to produce the adrenoleukodystrophy protein (ALDP), which is responsible for transporting specific molecules, such as very long-chain fatty acids (VLCFA), to peroxisomes, specialized compartments in cells where these molecules are broken down.
- Adrenomyeloneuropathy can be inherited from parent to child, and its inheritance follows an X-linked recessive pattern.
- The Adrenomyeloneuropathy symptoms can vary greatly from person to person. Common signs include leg stiffness, weakness, and pain, which often develop slowly and may intensify over time. The condition can also impact the nerves that control bladder, bowel, and sexual function, leading to various complications.
- Adrenomyeloneuropathy diagnosis requires a combination of biochemical, genetic, and imaging tests to confirm the condition and assess the extent of disease involvement. Since Adrenomyeloneuropathy symptoms can overlap with other neurological and metabolic disorders, a structured diagnostic approach is essential to ensure early and accurate detection.
- Currently, there is no treatment for Adrenomyeloneuropathy but there are promising clinical studies researching options. Treatment is unique to each person but generally focuses on alleviating symptoms of the disease. Steroid replacement therapy is available for treating adrenal insufficiency and generally improves the patient's quality of life.
- With no approved treatments for Adrenomyeloneuropathy, emerging therapies in late-stage and mid-stage development offer hope for patients. According to DelveInsight's estimates, potential drugs expected to drive significant change in the forecast period include MIN-102 (Leriglitazone), SBT101, VK0214, and others, bringing much-needed therapeutic options closer to reality.
- In December 2024, Bionomics Limited announced that positive results from its Phase 2 ATTUNE study have been published in NEJM Evidence. The data were also presented at the 63rd Annual Meeting of the American College of Neuropsychopharmacology (ACNP) during the inaugural “Promising Targets” session.
- In October 2024, Spur Therapeutics announced the publication of preclinical proof-of-concept data for SBT101, its first-in-class gene therapy for Adrenomyeloneuropathy, in Molecular Therapy Methods & Clinical Development. The findings reinforce SBT101’s potential as a groundbreaking treatment for Adrenomyeloneuropathy.
- In October 2024, Viking announced positive data from the company’s Phase Ib clinical trial of VK0214.
- The United States accounted for the largest Adrenomyeloneuropathy market size among the 7MM, around 45% of the total market size.
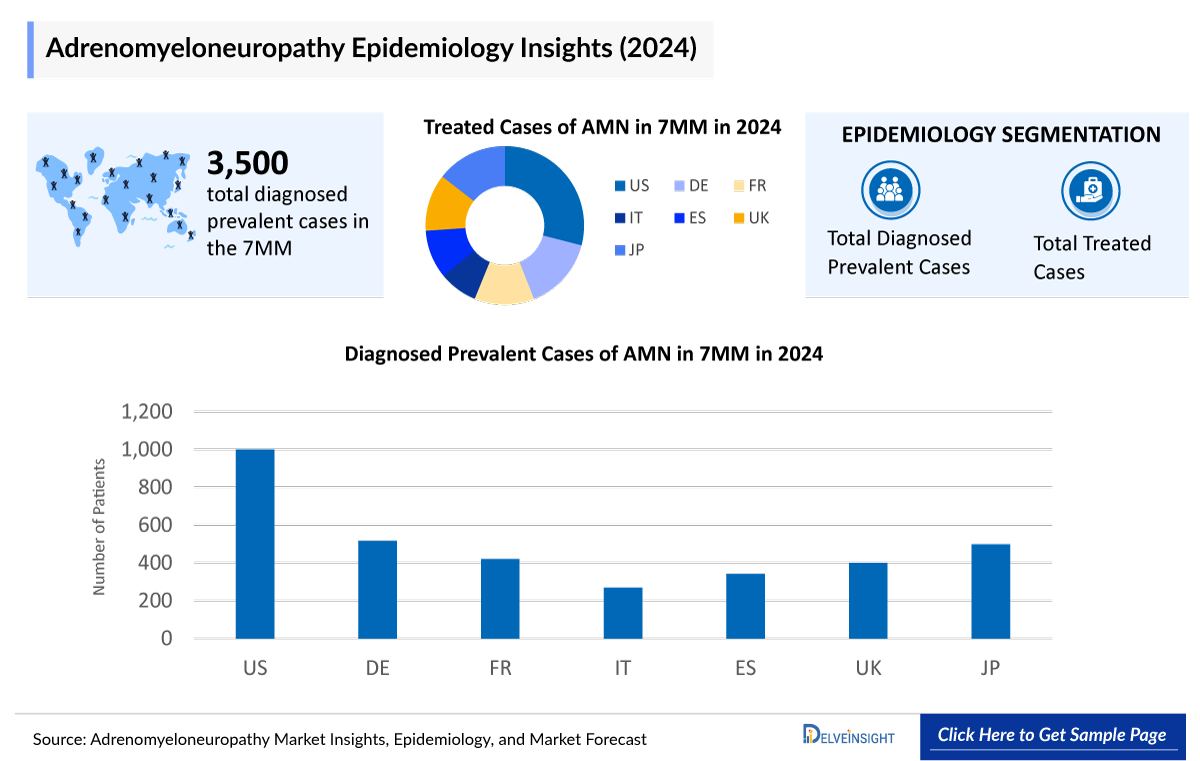
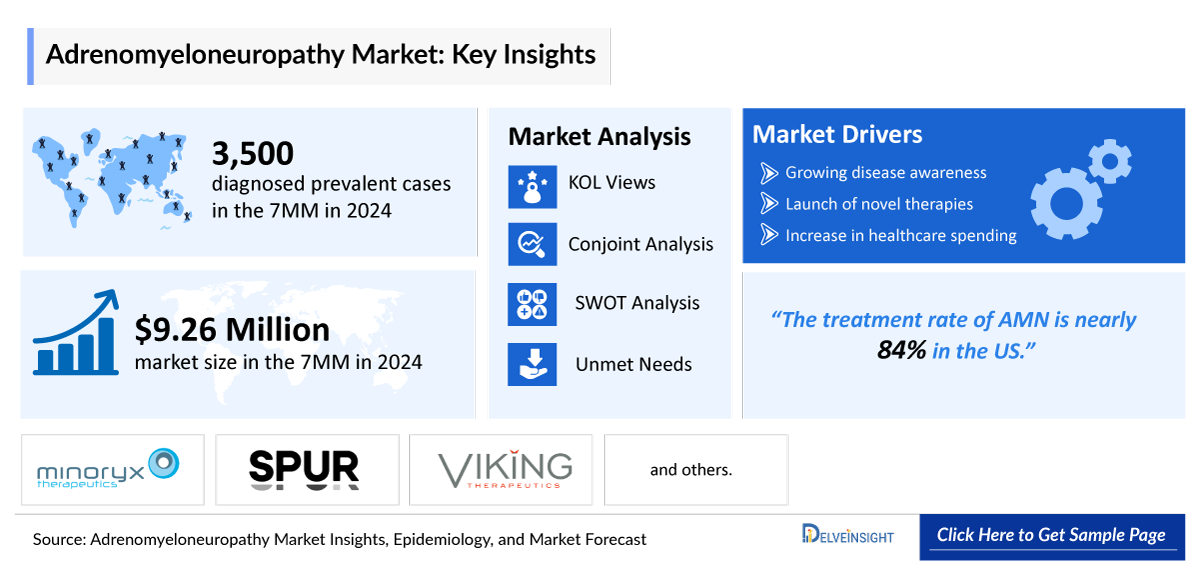
- In 2024, the diagnosed prevalent cases of Adrenomyeloneuropathy were approximately 3,500 cases in the 7MM, which will increase by 2034.
- In the 7MM, the highest number of diagnosed prevalent cases of Adrenomyeloneuropathy was observed in the US.
- Among EU4 and the UK, in 2024, Germany accounted for the highest number of Adrenomyeloneuropathy cases, followed by France, whereas Italy accounted for the lowest number of diagnosed prevalent cases.
- The Adrenomyeloneuropathy disease market growth is expected to be mainly driven by the increase in prevalence, less competitive scenarios, and increased initiatives to create public awareness and knowledge of the disease.
Key factors driving the Adrenomyeloneuropathy market
Rising Prevalence of Adrenomyeloneuropathy
Adrenomyeloneuropathy remains a rare but significant condition within the spectrum of X-linked adrenoleukodystrophy. In 2024, the US reported around 1K diagnosed cases, while Germany accounted for more than 27% of diagnosed prevalent cases among the EU4 and the UK. The disease’s impact varies by individual, with treatment often tailored to manage symptoms and improve quality of life. Steroid replacement therapy is commonly used to address adrenal insufficiency, offering meaningful benefits in patient care.
Promising Clinical Studies in Adrenomyeloneuropathy
Ongoing clinical research is actively exploring disease-modifying options for AMN, aiming to move beyond symptomatic management. Several mid- and late-stage clinical trials are showing encouraging progress, signaling a shift toward therapies that could address the underlying disease mechanisms.
Emerging Adrenomyeloneuropathy Therapies
The pipeline for AMN is gaining momentum, with late-stage and mid-stage candidates such as MIN-102 (Minoryx Therapeutics), SBT101 (Spur Therapeutics), and VK0214 (Viking Therapeutics) leading the way. These emerging therapies hold the potential to transform treatment standards and provide much-needed hope for patients. Key players, including Minoryx, Spur, and Viking Therapeutics, are expected to shape the future market landscape through innovative clinical development.
Report Summary
- The report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies, and the elaborate profiles of late-stage (Phase III, Phase II & Phase I) and prominent therapies that would impact the current treatment landscape and result in an overall market shift have been provided in the report.
- The report also encompasses a comprehensive analysis of the Adrenomyeloneuropathy market, providing an in-depth examination of its historical and projected market size (2020–2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journeys, and treatment preferences that help shape and drive the 7MM Adrenomyeloneuropathy market.
The table given below further depicts the key segments provided in the report
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Adrenomyeloneuropathy Epidemiology |
Segmented by:
|
|
Adrenomyeloneuropathy Market |
Segmented by:
|
|
Adrenomyeloneuropathy Market Analysis |
|
Adrenomyeloneuropathy Drug Chapters
The section dedicated to drugs in the Adrenomyeloneuropathy report provides an in-depth evaluation of late-stage Adrenomyeloneuropathy pipeline drugs (Phase III and Phase II). The drug chapters section provides valuable information on various aspects related to Adrenomyeloneuropathy clinical trials, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Adrenomyeloneuropathy.
Adrenomyeloneuropathy Emerging Therapies
Leriglitazone (MIN-102): Minoryx Therapeutics
Leriglitazone (MIN-102) is an investigational novel, orally bioavailable, and selective PPAR gamma agonist with a superior profile for CNS-related diseases.
Minoryx Therapeutics has announced the publication of data from its Phase II/III ADVANCE trial evaluating leriglitazone in male patients with adrenomyeloneuropathy. While the trial did not meet its primary endpoint, the results demonstrated promising signs of efficacy. Leriglitazone showed potential in stabilizing disease progression, with improvements in body sway and positive effects on key biomarkers. Notably, the treatment led to a reduction in neurofilament light levels and helped preserve blood-brain barrier integrity, indicating a potential neuroprotective effect.
In November 2022, Minoryx Therapeutics and Neuraxpharm completed a strategic license agreement granting Neuraxpharm exclusive European rights to leriglitazone, a brain-penetrant PPARγ agonist. Neuraxpharm will commercialize leriglitazone in Europe and collaborate on its development, while Minoryx retains rights in the US and other regions, excluding China
Liraglutide received Fast Track Designation from the FDA for the treatment of all forms of X-ALD, including Adrenomyeloneuropathy and cCALD.
SBT101: Spur Therapeutics
SBT101 is the first investigational AAV-based gene therapy developed to counteract ABCD1 mutations in adrenomyeloneuropathy (Adrenomyeloneuropathy). It aims to restore ABCD1 expression and reduce very long-chain fatty acid (VLCFA) levels, targeting the underlying cause of the disease. Using an adeno-associated virus (AAV) capsid, SBT101 delivers a functional ABCD1 gene directly to spinal cord cells to restore cellular function and slow disease progression.
Preclinical studies have shown dose-dependent improvements in Adrenomyeloneuropathy disease markers in mouse models and favorable tolerability in non-human primates at six months post-treatment. SBT101 also demonstrated increased ABCD1 expression and reductions in toxic substrates in the spinal cord.
SBT101 is currently being evaluated in a Phase I/II first-in-human clinical trial.
|
Comparison of Emerging Therapies | |||||||
|
Product |
Company |
Phase |
Indication |
Designation |
RoA |
MoA |
Molecule Type |
|
Leriglitazone (MIN-102) |
Minoryx Therapeutics |
II/III |
Adrenomyeloneuropathy |
US: FTD
|
Oral |
Peroxisome proliferator-activated receptor gamma agonists |
Small molecule |
|
SBT101 |
Spur Therapeutics |
I/II |
Adrenomyeloneuropathy |
US: FTD, ODD |
IV |
ALDP expression stimulants |
Gene therapy |
|
VK0214 |
Viking Therapeutics |
I |
Adrenomyeloneuropathy |
NA |
Oral |
Thyroid hormone receptor beta agonists |
Small molecule |
Adrenomyeloneuropathy Market Outlook
The Adrenomyeloneuropathy market is gradually evolving, though current pipeline activity remains relatively limited, particularly given the high unmet needs among patients. Key industry players, including Minoryx Therapeutics, Spur Therapeutics, Viking Therapeutics, and others, are actively advancing their lead candidates through various stages of clinical development.
Despite this progress, the Adrenomyeloneuropathy market faces several hurdles, including a general lack of disease awareness, reimbursement limitations, and ongoing concerns about the efficacy of available Adrenomyeloneuropathy treatments. However, the market is expected to expand over the coming years, driven by a growing patient population, increased research funding, and continuous advancements in therapeutic options. Further momentum is likely to come from improved diagnostic techniques, preventive strategies, and efforts to enhance patient education and awareness.
Recent studies highlight the considerable treatment burden faced by Adrenomyeloneuropathy patients. A significantly higher percentage of Adrenomyeloneuropathy patients were found to use prescription medications compared to control groups (89.1% vs. 69.5%), with 82.5% taking drugs specifically targeting Adrenomyeloneuropathy-related symptoms, compared to 50.9% in controls. The most commonly prescribed medications included those for adrenal insufficiency (60.7%), pain management (42.2%), incontinence (30.4%), musculoskeletal issues (27.1%), antidepressants and anti-anxiety medications (both at 25.7%), and anticonvulsants (25.4%). Additionally, Adrenomyeloneuropathy patients filled notably more prescriptions annually than controls, especially for adrenal insufficiency, anticonvulsants, and musculoskeletal support—highlighting the substantial medical needs within this population.
Geographically, the United States holds the largest Adrenomyeloneuropathy market share, followed by the EU4 countries, the UK, and Japan. This market leadership is supported by growing awareness, favorable government policies, funding initiatives, regulatory approvals, and emerging research. These factors, combined with targeted awareness campaigns and stronger patient advocacy, are expected to drive significant growth in the Adrenomyeloneuropathy market through the forecast period of 2025–2034.
Further details are provided in the report…
Adrenomyeloneuropathy Disease Understanding and Treatment
Adrenomyeloneuropathy Overview
Adrenomyeloneuropathy is the most common form of adrenoleukodystrophy (ALD), primarily affecting adult males. It typically presents with a gradual onset of symptoms such as leg stiffness, muscle weakness, gait disturbances, and nerve-related pain or discomfort. As the disease progresses, individuals may also experience bladder and bowel dysfunction, along with sexual dysfunction. Unlike cerebral Adrenomyeloneuropathy, which can lead to rapid cognitive decline, Adrenomyeloneuropathy generally progresses more slowly and is less likely to impair cognitive function. However, it can still lead to considerable physical disability over time.
Clinically, Adrenomyeloneuropathy can be classified into two subtypes: Adrenomyeloneuropathy with cerebral involvement, where both the spinal cord and brain are affected, and Adrenomyeloneuropathy without cerebral involvement, where the disease remains confined to the spinal cord. Among those diagnosed with Adrenomyeloneuropathy, around 54% retain normal brain function, while approximately 46% develop varying degrees of cerebral involvement. This distinction is important for prognosis and treatment planning, as cerebral forms may require more intensive monitoring and intervention.
Further details are provided in the report…
Adrenomyeloneuropathy Diagnosis
Adrenomyeloneuropathy can be challenging to diagnose accurately and often takes years, primarily because many primary care providers are unfamiliar with the condition. Patients typically consult multiple specialists before receiving a definitive diagnosis. Due to the overlap of Adrenomyeloneuropathy symptoms with other neurological and metabolic disorders, a structured and comprehensive diagnostic approach is essential. This includes a combination of biochemical, genetic, and imaging tests to confirm the presence of the disease and determine its extent.
The Adrenomyeloneuropathy diagnostic process usually begins with a blood test to measure very long-chain fatty acid (VLCFA) levels. Elevated concentrations of VLCFAs, particularly C26:0 and the ratios of C24:0/C22:0 and C26:0/C22:0, are key biochemical markers indicative of Adrenomyeloneuropathy. While these findings can strongly suggest the disorder, genetic testing is crucial for confirmation, as it provides the definitive diagnosis. Additionally, since Adrenomyeloneuropathy primarily affects the spinal cord and sometimes the brain, MRI scans are important for detecting characteristic neurodegenerative changes associated with the disease.
Further details related to country-based variations are provided in the report…
Adrenomyeloneuropathy Treatment
Adrenomyeloneuropathy treatment focuses on symptom management and disease monitoring, as no curative therapy currently exists. A multidisciplinary approach is essential, involving neurologists, endocrinologists, and rehabilitation specialists. Neurological care includes regular MRI monitoring for cerebral ALD and symptom management for spastic paraparesis, neuropathic pain, and bladder dysfunction. Endocrinological care is critical, with annual adrenal function screening and steroid replacement therapy for those with adrenal insufficiency. Supportive therapies such as physical and occupational therapy help maintain mobility, while medications can alleviate spasticity and pain. Experimental approaches, including gene therapy and novel pharmacological interventions, are under investigation to modify disease progression. Early diagnosis and proactive management are key to improving the quality of life in individuals with Adrenomyeloneuropathy.
Further details related to treatment and management are provided in the report…
Adrenomyeloneuropathy Epidemiology
The Adrenomyeloneuropathy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases, and total treated cases in the United States, EU4 countries (Germany, France, Italy, Spain), the United Kingdom, and Japan from 2020 to 2034.
- The total diagnosed cases of Adrenomyeloneuropathy in the US were around 1,000 cases in 2024.
- Germany had the highest diagnosed prevalent cases of Adrenomyeloneuropathy among the EU4 and the UK, accounting for more than 27% of cases in 2024.
- In 2024, the total treated cases of Adrenomyeloneuropathy in Japan were around 433.
Further details related to epidemiology will be provided in the report…
KOL Views on Adrenomyeloneuropathy
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Adrenomyeloneuropathy, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 15 KOLs across the 7MM. We contacted institutions such as the University of Tsukuba, Duke University, University of Glasgow, Washington University School of Medicine, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Adrenomyeloneuropathy market, which will assist our clients in analyzing the overall epidemiology and market scenario.
The opinions of experts from various regions have been provided below:
“In its early stages, Adrenomyeloneuropathy can present with neuropathic symptoms, typically treated with analgesics, anti-spasmodics, and botulinum toxins, as well as with bladder symptoms, managed initially with lifestyle changes. When adrenal insufficiency is identified, corticosteroid replacement therapy is essential and can be lifesaving.” - MD, Germany
“Heterozygous females are not at increased risk to develop CALD, but are at increased risk to develop Adrenomyeloneuropathy and primary adrenocortical insufficiency with increasing age.” - MD, US
Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated, and based on these measures, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Adrenomyeloneuropathy Market Report Insights
- Adrenomyeloneuropathy Patient Population
- Adrenomyeloneuropathy Therapeutic Approaches
- Adrenomyeloneuropathy Market Size
- Adrenomyeloneuropathy Market Trends
- Existing Adrenomyeloneuropathy Market Opportunity
Adrenomyeloneuropathy Market Report Key Strengths
- Ten-year Forecast
- The 7MM Coverage
- Adrenomyeloneuropathy Epidemiology Segmentation
- Key Cross Competition
Adrenomyeloneuropathy Market Report Assessment
- Current Adrenomyeloneuropathy Treatment Practices
- Reimbursements
- Adrenomyeloneuropathy Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
Key Questions
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Adrenomyeloneuropathy management recommendations?
- Would research and development advances pave the way for future tests and therapies for Adrenomyeloneuropathy?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Adrenomyeloneuropathy?
- What kind of uptake will the new therapies witness in the coming years in Adrenomyeloneuropathy patients?

.png)