Angioimmunoblastic T-cell Lymphoma (AICL) Market
Key Highlights:
- The Angioimmunoblastic T-cell lymphoma market is anticipated to witness a substantial positive shift owing to better uptake of existing Angioimmunoblastic T-cell lymphoma drugs market, expected market launch of Angioimmunoblastic T-cell lymphoma therapies, and raised awareness.
- Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive form of peripheral T-cell lymphoma (PTCL) characterized by high fever, generalized lymphadenopathy, and systemic involvement, often accompanied by autoimmune disorders.
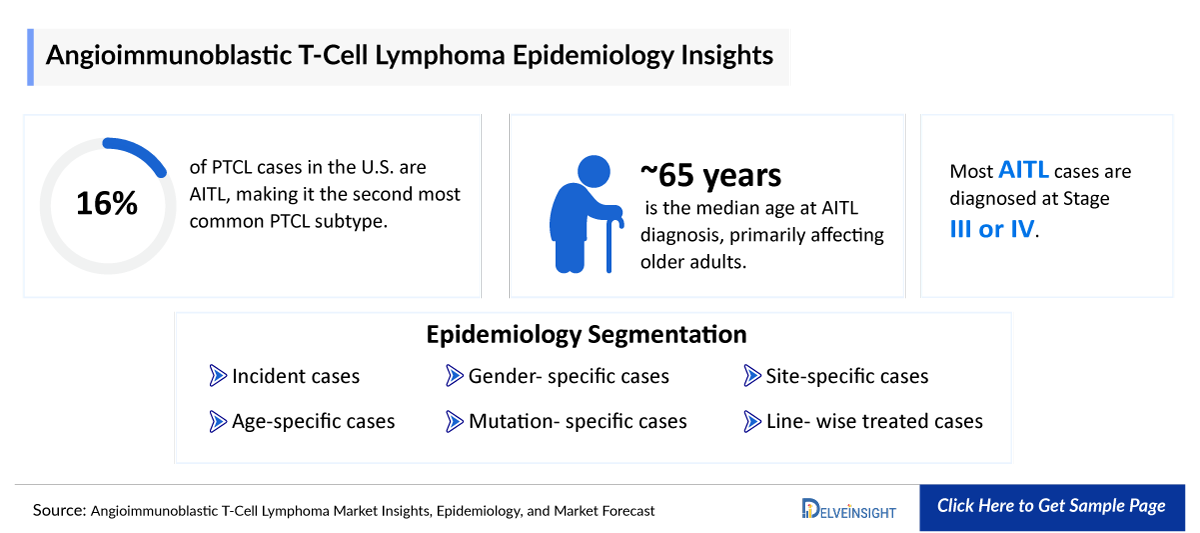
- AITL ranks as the second most prevalent subtype of PTCL in the US, constituting approximately 16% of PTCL cases.
- Among the seven major markets, EU4 and the UK accounts for the largest Angioimmunoblastic T-cell lymphoma market size, followed by US and the Japan.
- In 2009, FOLOTYN (pralatrexate) became the first drug to be approved by the FDA for the treatment of relapsed or refractory PTCL; patients with AITL were included in the clinical study that supported this approved use.
- In 2018, ADCETRIS (brentuximab vedotin), received an indication expansion and got approved by the FDA for patients with CD30-expressing PTCL, including AITL, in combination with cyclophosphamide, doxorubicin, and prednisone
- Potential emerging Angioimmunoblastic T-cell lymphoma therapies include- AUTO4, ST-001, ARV-393, and others.
DelveInsight's “Angioimmunoblastic T-cell lymphoma (AITL) – Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Angioimmunoblastic T-cell lymphoma (AITL) , historical and forecasted epidemiology as well as the Angioimmunoblastic T-cell lymphoma (AITL) market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Angioimmunoblastic T-cell lymphoma (AITL) market report provides real-world prescription pattern analysis, emerging Angioimmunoblastic T-cell lymphoma drugs, market share of individual therapies, and historical and forecasted 7MM Angioimmunoblastic T-cell lymphoma (AITL) market size from 2020 to 2034. The report also covers current Angioimmunoblastic T-cell lymphoma (AITL) Angioimmunoblastic T-cell lymphoma treatment market/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Angioimmunoblastic T-cell lymphoma (AITL) Understanding and Treatment Algorithm
Angioimmunoblastic T-cell lymphoma (AITL) Overview, Country-Specific Treatment Guidelines and Diagnosis
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive form of peripheral T-cell lymphoma. It develops from a particular type of white blood cell called a follicular helper T cell Conventional karyotyping shows clonal abnormalities in approximately 30-50% of AITL cases, with the most common abnormalities including gain of chromosomes 5q, 21, and 3q, concurrent trisomies of 5 and 21, and loss of 6q recent advances in genomics have identified recurrent mutations in genes such as TET2, DNMT3A, IDH2, and RHOA, which are important for diagnosis and prognosis.
The Diagnosis starts with routine testing, such as complete blood count (CBC), complete metabolic panel, lactate dehydrogenase (LDH) level, testing for EBV, hepatitis B and C, HIV and human T-lymphotropic virus. Laboratory findings in AITL include cytopenias, elevated inflammatory markers, LDH, positive autoimmune phenomena (positive coombs’ test, thyroid dysfunction, etc.) and polyclonal hypergammaglobulinemia. Bone marrow biopsy and PET/computed tomography imaging are needed for staging.
Angioimmunoblastic T-cell lymphoma (AITL) Treatment
The front-line treatment typically involves a multiagent chemotherapy regimen such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and consideration for autologous stem-cell transplant (SCT). In the relapsed and refractory settings, allogeneic SCT offers the chance for long-term remission. Current Treatment Options are- Steroids, Multiagent Chemotherapy and Targeted Therapies.
Further details related to diagnosis and treatment are provided in the report
Angioimmunoblastic T-cell lymphoma (AITL) Epidemiology
The Angioimmunoblastic T-cell lymphoma epidemiology chapter in the report provides historical as well as forecasted epidemiology in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Angioimmunoblastic T-cell lymphoma (AITL) epidemiology is segmented with detailed insights into incident cases, age- specific cases, gender- specific cases, mutation- specific cases, site-specific cases, and line-wise treated cases of Angioimmunoblastic T-cell lymphoma (AITL).
- AITL represents only 1% to 2% of all cases non-Hodgkin lymphoma (NHL) cases.
- As per DelveInsight’s estimate, AITL primarily affects older individuals, with the median age of diagnosis being 65 years.
- Although, a new prognostic tool (AITL score) was recently developed to predict several measures of survival in patients with AITL, still the overall prognosis for AITL remains poor with a 5-year median survival of ~32%.
- Majority of the diagnosed AITL cases fall under Stage-III and Stage-IV grade.
Angioimmunoblastic T-cell lymphoma (AITL) Drug Chapter
The drug chapter segment of the Angioimmunoblastic T-cell lymphoma (AITL) report encloses a detailed analysis of Angioimmunoblastic T-cell lymphoma (AITL) marketed Angioimmunoblastic T-cell lymphoma drugs and late-stage (Phase III and Phase II) Angioimmunoblastic T-cell lymphoma pipeline drugs. It also deep dives into the Angioimmunoblastic T-cell lymphoma (AITL) pivotal Angioimmunoblastic T-cell lymphoma clinical trials details, recent and expected market approvals, patent details, the latest Angioimmunoblastic T-cell lymphoma news, and recent deals and collaborations.
Marketed Angioimmunoblastic T-cell lymphoma Drugs
ADCETRIS: Seattle Genetics
ADCETRIS (Brentuximab vedotin) is a CD30-directed antibody-drug conjugate indicated for the treatment of adult patients with previously untreated systemic anaplastic large cell lymphoma (sALCL) or other CD30-expressing peripheral Tcell lymphomas (PTCL), AITL and PTCL not otherwise specified, in combination with cyclophosphamide, doxorubicin, and prednisone.
Brentuximab vedotin is an antibody-drug conjugate. The antibody is a chimeric IgG1 directed against CD30. The small molecule, Monomethyl auristatin E (MMAE), is a microtubule disrupting agent. MMAE is covalently attached to the antibody via a linker. Nonclinical data suggest that the anticancer activity of ADCETRIS is due to the binding of the ADC to CD30-expressing cells, followed by internalization of the ADC-CD30 complex, and the release of MMAE via proteolytic cleavage. Binding of MMAE to tubulin disrupts the microtubule network within the cell, subsequently inducing cell cycle arrest and apoptotic death of the cells.
Note: Detailed current therapies assessment will be provided in the full report of Angioimmunoblastic T-cell lymphoma (AITL)
Emerging Angioimmunoblastic T-cell lymphoma Drugs
AUTO4: Autolus Therapeutics
AUTO4 a CAR T cell treatment targeting TRBC1 in patients with relapsed or refractory TRBC1 positive selected T-Non-Hodgkin Lymphoma including AITL.
The study will consist of 2 phases, a Phase I/dose escalation phase and a Phase II/expansion phase. Patients with relapsed or refractory TRBC1 positive selected T-NHL will be enrolled in both phases of the study. Eligible patients will undergo leukapheresis to harvest T cells, the starting material for the manufacture of the autologous CAR-T product AUTO4. Following preconditioning by a chemotherapeutic regimen, the patient will receive AUTO4 intravenously as a single dose following which they will then enter a 24-month follow-up period.
ST-001: SciTech Development
ST-001 nanoFenretinide is SciTech Development's lead drug candidate, a patented nanoparticle formulation that combines their proprietary Drug Delivery Platform (SDP) with the drug fenretinide.
ST-001 is currently in a Phase I Angioimmunoblastic T-cell lymphoma clinical trials for the treatment of relapsed/refractory T-cell non-Hodgkin lymphoma including AITL. The Angioimmunoblastic T-cell lymphoma Mechanism of Action involves a dual-pronged approach that restores tumor immunity and induces apoptosis in cancer cells.
Note: Detailed emerging therapies assessment will be provided in the final report.
Angioimmunoblastic T-cell lymphoma (AITL) Market Outlook
Key Angioimmunoblastic T-cell lymphoma players, such as Autolus Therapeutics, SciTech Development, Arvinas, and others are evaluating their lead candidates in different stages of clinical development for the Angioimmunoblastic T-cell lymphoma treatment.
Short-course combinations for AITL include historical treatments adapted from relapsed aggressive B-cell lymphomas. These include classic inpatient regimens like ICE (ifosphamide, carboplatin, etoposide), DHAP (dexamethasone, cytarabine, cisplatinum), and ESHAP (etoposide, methylprednisolone, cisplatinum, cytarabine), as well as outpatient regimens like Gem-P (gemcitabine, cisplatinum, methylprednisolone), GDP (gemcitabine, cisplatinum, dexamethasone), and bendamustine.
Combination chemotherapy regimens given inpatient have been shown to have higher response rates.
The combination potential for Angioimmunoblastic T-cell lymphoma treatment is expanding with emerging signals suggesting the possible use of JAK-2 inhibitors, hypo-methylating agents, and IDH2 inhibitors, all currently entering Angioimmunoblastic T-cell lymphoma clinical trials.
Angioimmunoblastic T-cell lymphoma (AITL) Drugs Uptake
This section focuses on the uptake rate of potential Angioimmunoblastic T-cell lymphoma drugs market expected to be launched in the Angioimmunoblastic T-cell lymphoma treatment market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Angioimmunoblastic T-cell lymphoma companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Angioimmunoblastic T-cell lymphoma (AITL) Activities
The report provides insights into different therapeutic candidates in Phase II and Phase I stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Angioimmunoblastic T-cell lymphoma (AITL) emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging Angioimmunoblastic T-cell lymphoma treatment market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Angioimmunoblastic T-cell lymphoma (AITL). This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Angioimmunoblastic T-cell lymphoma treatment market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers’ concerns about budget impact. The high cost of rare disease drugs usually has a limited effect on the budget due to the small number of eligible patients being prescribed the drug. The US FDA has approved several rare disease therapies in recent years. From a patient perspective, health insurance and payer coverage guidelines surrounding rare disease treatments restrict broad access to these treatments, leaving only a small number of patients who can bypass insurance and pay for products independently.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of Angioimmunoblastic T-cell lymphoma (AITL), explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Angioimmunoblastic T-cell lymphoma therapeutics market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Angioimmunoblastic T-cell lymphoma (AITL) market.
Angioimmunoblastic T-cell lymphoma (AITL) Report Insights
- Patient Population
- Therapeutic Approaches
- Angioimmunoblastic T-cell lymphoma (AITL) Pipeline Analysis
- Angioimmunoblastic T-cell lymphoma (AITL) Market Size and Trends
- Existing and future Angioimmunoblastic T-cell lymphoma therapeutics market Opportunity
Angioimmunoblastic T-cell lymphoma (AITL) Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Angioimmunoblastic T-cell lymphoma (AITL) Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Angioimmunoblastic T-cell lymphoma Drugs Market and Key Angioimmunoblastic T-cell lymphoma Market Forecast Assumptions
Angioimmunoblastic T-cell lymphoma (AITL) Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM Angioimmunoblastic T-cell lymphoma therapeutics market?
- What was the Angioimmunoblastic T-cell lymphoma (AITL) total Angioimmunoblastic T-cell lymphoma market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label Angioimmunoblastic T-cell lymphoma therapies?
- How would the Angioimmunoblastic T-cell lymphoma market drivers, barriers, and future opportunities affect the Angioimmunoblastic T-cell lymphoma market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Angioimmunoblastic T-cell lymphoma (AITL)?
- How many Angioimmunoblastic T-cell lymphoma companies are developing therapies for the treatment of Angioimmunoblastic T-cell lymphoma (AITL)?
- What are the recent novel therapies, targets, Angioimmunoblastic T-cell lymphoma mechanism of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Angioimmunoblastic T-cell lymphoma treatment Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Angioimmunoblastic T-cell lymphoma market landscape in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Angioimmunoblastic T-cell lymphoma therapeutics market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Angioimmunoblastic T-cell lymphoma market so that the upcoming players can strengthen their development and launch strategy.
Get access to our insightful blogs @ DelveInsight Blogs