Autosomal Dominant Polycystic Kidney Disease Market
-
The Autosomal Dominant Polycystic Kidney Disease Treatment Market is expected to strengthen as awareness of the disease increases and more effective interventions are being developed.
-
The continual increase in autosomal dominant polycystic kidney disease diagnosed cases, driven by technological advancements facilitating early detection, presents a fertile ground for the introduction of novel therapies in the autosomal dominant polycystic kidney disease market. Furthermore, ongoing progress in understanding the genetic underpinnings, non-invasive monitoring, and prognostication of autosomal dominant polycystic kidney disease promises enhanced disease management, with pre-symptomatic diagnosis offering further avenues for patient care optimization.
-
With limited US FDA-approved therapies, there is an opportunity for key players to introduce disease-modifying therapies addressing the root causes of cyst formation and kidney deterioration for autosomal dominant polycystic kidney disease.
- There is a profound effect of autosomal dominant polycystic kidney disease on patients' well-being, citing chronic pain, kidney impairment, and related health issues. They underscore the necessity of addressing both the physical manifestations and the psychological toll of enduring a chronic, advancing condition.
Request for unlocking the CAGR of the "Autosomal Dominant Polycystic Kidney Disease Drugs Market"
DelveInsight’s report titled “Autosomal Dominant Polycystic Kidney Disease Drugs Market Insights, Epidemiology, and Market Forecast – 2034” comprehensively analyzes autosomal dominant polycystic kidney disease. The report provides a comprehensive analysis of historical and projected epidemiological data, covering total diagnosed prevalent cases of autosomal dominant polycystic kidney disease, total age-specific diagnosed prevalent cases of autosomal dominant polycystic kidney disease, and total mutation-specific cases of autosomal dominant polycystic kidney disease in the 7MM.
The Autosomal Dominant Polycystic Kidney Disease Treatment Market Size Report provides a comprehensive insight into different facets concerning the patient population, encompassing diagnosis, prescribing trends, physician viewpoints, market accessibility, therapy, and forthcoming market advancements across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan spanning from 2020 to 2034.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Autosomal Dominant Polycystic Kidney Disease Epidemiology |
|
|
Autosomal Dominant Polycystic Kidney Disease Drugs Market |
|
|
Autosomal Dominant Polycystic Kidney Disease Drugs Market Analysis |
|
|
Autosomal Dominant Polycystic Kidney Disease Companies |
|
|
Autosomal Dominant Polycystic Kidney Disease Market Growth Barriers |
A significant barrier to the growth of the autosomal dominant polycystic kidney disease market is the high cost associated with developing and accessing innovative therapies. Additionally, regulatory hurdles and the complexity of clinical trials for rare diseases like autosomal dominant polycystic kidney disease pose challenges for companies aiming to bring new treatments to market. |
Autosomal Dominant Polycystic Kidney Disease Treatment Market Landscape
Autosomal dominant polycystic kidney disease, also called “adult PKD” is the most common inherited kidney disorder characterized by the growth of cysts in the kidneys, which eventually leads to kidney failure. A monogenetic disorder, autosomal dominant polycystic kidney disease is caused by mutations in either the PKD1 gene found on chromosome 16 or the PKD2 gene found on chromosome 4. Mutations in PKD1 are more common and account for about 85% of all autosomal dominant polycystic kidney disease cases.
Autosomal dominant polycystic kidney disease is characterized by bilateral renal cysts, kidney pain, frequent urinary tract infection, hematuria, nephrolithiasis, hypertension, and progressive renal failure due to progressive enlargement of cysts and fibrosis. It is one of the leading causes of renal replacement and end-stage renal disease.
Autosomal Dominant Polycystic Kidney Disease Diagnosis
Diagnosing autosomal dominant polycystic kidney disease usually entails a multifaceted approach, including assessing symptoms, conducting imaging tests, genetic analysis, and reviewing family medical history. Urine examinations are conducted to detect the presence of blood or protein, while imaging techniques like ultrasound are employed to visualize kidney abnormalities. Additionally, glomerular filtration rate (GFR) testing may be performed to assess kidney function. This comprehensive diagnostic process aims to accurately identify autosomal dominant polycystic kidney disease, enabling prompt intervention and management strategies.
Further details related to country-based variations are provided in the report…
Autosomal Dominant Polycystic Kidney Disease Treatment
There is currently no cure for autosomal dominant polycystic kidney disease. However, extensive research is being conducted. Recent research suggests that drinking plain water throughout the day and avoiding caffeine-containing beverages can help slow cyst growth. Research is also assisting us in better understanding the genetic basis of autosomal dominant polycystic kidney disease.
Individuals with autosomal dominant polycystic kidney disease are treated by paying close attention to diet, fluid intake, blood pressure control, and avoiding harmful drug and lifestyle choices. Also, inhibition of the RAAS using angiotensin-converting enzyme (ACE) inhibitors is favored, as well as the use of angiotensin-receptor blockers (ARBs) and β-blockers.
The findings from the early studies with small cohorts of patients demonstrated that ACE inhibitors reduce the severity of proteinuria and left ventricular mass compared to diuretics and calcium channel blockers. In contrast, ARBs resulted in a greater reduction in proteinuria than calcium channel blockers.
Currently, there is only one approved therapy for the treatment of autosomal dominant polycystic kidney disease, i.e., tolvaptan, which is a vasopressin V2 receptor antagonist. It is the first drug treatment available to slow kidney function decline in adults at risk of rapidly progressing autosomal dominant polycystic kidney disease.
But this comes with a black box warning as well. It is mentioned on the label that Jynarque (tolvaptan) can cause serious and potentially fatal liver injury. In addition, acute liver failure requiring liver transplantation has been reported in some patients. However, novel strategies to limit cyst burden have provided encouraging results, but the treatment of hypertension and proteinuria remains the main idea for the medical management of patients. Additionally, affected individuals and their families may benefit from genetic counseling.
Autosomal Dominant Polycystic Kidney Disease Epidemiology
As the market is derived using a patient-based model, the autosomal dominant polycystic kidney disease epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of autosomal dominant polycystic kidney disease, total age-specific diagnosed prevalent cases of autosomal dominant polycystic kidney disease, and total mutation-specific cases of autosomal dominant polycystic kidney disease in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
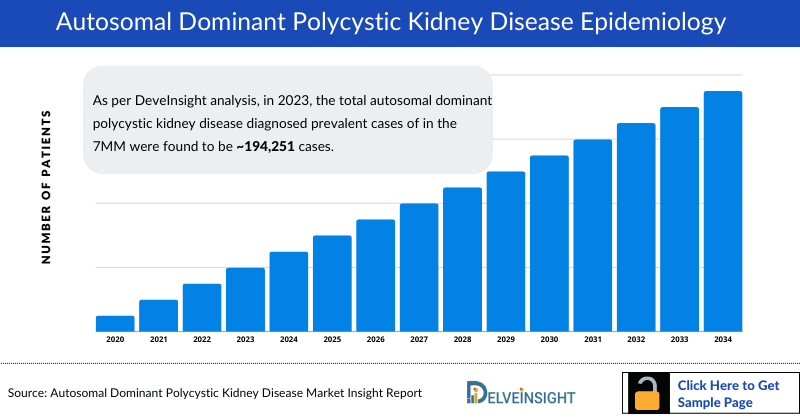
- As per DeveInsight analysis, in 2023, the total autosomal dominant polycystic kidney disease diagnosed prevalent cases of in the 7MM were found to be approximately 194,251 cases. These cases are likely to change by 2034 in the forecast period 2024-2034.
- The US accounted for approximately 144,697 autosomal dominant polycystic kidney disease diagnosed prevalent cases in the year 2023. These cases are expected to increase driven by the increasing occurrence of kidney disorders due to obesity and hypertension in the US.
- In 2023, among the EU4 and the UK, the UK accounted for the highest number of autosomal dominant polycystic kidney disease diagnosed prevalent cases with approximately 68,138 cases followed by France at 61,676. In contrast, Italy with nearly 16,476 cases accounts for the lowest number of cases.
- In the US, the age-specific cases of <5, 5–14, 15–24, 25–44, 45–64, ≥ 65 years of age for autosomal dominant polycystic kidney disease were 1,158, 4,052, 9,695, 45,580, 57,300, and 26,914 cases respectively in 2023, which are expected to by 2034.
- According to DelveInsight's analysis, Japan reported an estimated 30,930 cases of autosomal dominant polycystic kidney disease in 2023. Among these cases, approximately 26,290 were attributed to mutations in the PKD1 gene, while around 4,639 cases were associated with mutations in the PKD2 gene.
Autosomal Dominant Polycystic Kidney Disease Drugs Market Chapters
The Autosomal Dominant Polycystic Kidney Disease drugs market chapter segment of the autosomal dominant polycystic kidney disease market outlook report encloses a detailed analysis of autosomal dominant polycystic kidney disease-marketed drugs and late-stage (Phase III and Phase II) Autosomal Dominant Polycystic Kidney Disease pipeline drugs analysis. It also helps understand the autosomal dominant polycystic kidney disease clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Autosomal Dominant Polycystic Kidney Disease news and press releases.
Autosomal Dominant Polycystic Kidney Disease Marketed Drugs
- JYNARQUE/ JINARC/SAMSCA (tolvaptan): Otsuka America Pharmaceutical
JYNARQUE, developed by Otsuka Pharmaceutical, is a selective vasopressin V2-receptor antagonist to treat adults with autosomal dominant polycystic kidney disease. Tolvaptan inhibits vasopressin receptors in the kidneys and retards kidney function decline in adults at risk of rapidly progressing autosomal dominant polycystic kidney disease.
It has a 29 times higher affinity for V2-receptor than for V1a, and this is 1.8 times the affinity of natural arginine vasopressin (AVP) for V2, which helps regulate urine volume and osmolality, serum sodium levels, and free water clearance rate. It is conducting Phase III trials to evaluate the effect of tolvaptan on the need for renal replacement therapy in pediatric subjects (28 days to less than 12 weeks old at the time of enrollment) with autosomal dominant polycystic kidney disease.
Autosomal Dominant Polycystic Kidney Disease Emerging Drugs
- RGLS8429: Regulus Therapeutics
RGLS8429 is a novel, next-generation oligonucleotide for the treatment of autosomal dominant polycystic kidney disease designed to inhibit miR-17 and preferentially target the kidney. Administration of RGLS8429 has shown robust data in preclinical models, where clear improvements in kidney function, size, and other measures of disease severity and a superior pharmacologic profile, have been demonstrated along with a superior pharmacologic profile in preclinical studies compared to Regulus' first-generation compound.
The company is looking forward to the data from their third and fourth cohorts to inform further for the potentially pivotal Phase II trial design. Furthermore, the company held a Type D meeting with the US FDA to discuss the accelerated approval pathway. The meeting was constructive and confirmed the potential for an accelerated approval pathway based on a single Phase II study of RGLS8429 for the treatment of autosomal dominant polycystic kidney disease.
- XRx-008: Xortx Therapeutics
XRx-008 is being developed as a treatment of progressive kidney disease in autosomal dominant polycystic kidney disease. It is a proprietary oxypurinol formulation intended to be administered once daily to decrease uric acid production by xanthine oxidase, thereby decreasing chronic injury associated with progressing kidney disease in patients. Decreasing the production of uric acid is expected to reduce systemic and kidney inflammation, decrease the rate of initiation of cyst genesis and cyst growth, reverse endothelial dysfunction, decrease proteinuria, and decrease the rate of decline of kidney filtering capacity, all to the benefit of patients with autosomal dominant polycystic kidney disease.
Recently XORTX announced the publication of a research paper in the peer-reviewed American Journal of physiology titled Raising serum uric acid with an uricase inhibitor worsens PKD in rat and mouse models. Furthermore, the drug candidate is being investigated in a Phase II clinical trial to treat autosomal dominant polycystic kidney disease.
- VX-407: Vertex Pharmaceuticals
VX-407 is a first-in-class small molecule corrector that is designed to treat autosomal dominant polycystic kidney disease in patients with a subset of PKD1 variants, estimated at ~25,000 (or ~10%) of the overall ~250,000 autosomal dominant polycystic kidney disease patient population, by correcting defective PC1 folding to restore function. In doing so, the aim is to stop the growth of kidney cysts and reduce kidney volume, thereby preventing progression to kidney failure.
Recently the company posted FDA Clearance of Investigational New Drug Application for VX-407 in the treatment of autosomal dominant polycystic kidney disease. Furthermore, the drug candidate is being investigated in a Phase I trial.
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Autosomal Dominant Polycystic Kidney Disease Drugs Market Insights
Autosomal Dominant Polycystic Kidney Disease treatment market involves diverse approaches and medications. Renin-angiotensin-aldosterone system (RAAS) inhibitors like ACE inhibitors and ARBs are commonly used to manage hypertension and slow kidney disease progression. Vasopressin V2 receptor antagonists such as tolvaptan aim to inhibit cyst growth and maintain kidney function by blocking vasopressin action. mTOR inhibitors like everolimus target cell proliferation and cyst formation pathways, potentially slowing cyst growth. Additionally, CFTR modulators are under investigation for their ability to ameliorate cystic fibrosis-associated complications in ADPKD patients.
Autosomal Dominant Polycystic Kidney Disease Market Outlook
Autosomal dominant polycystic kidney disease also called “adult PKD” is the most common inherited kidney disorder characterized by the growth of cysts in the kidneys, which eventually leads to kidney failure. A monogenetic disorder, autosomal dominant polycystic kidney disease is caused by mutations in either the PKD1 gene found on chromosome 16 or the PKD2 gene found on chromosome 4.
Mutations in PKD1 are more common and account for about 85% of all Autosomal Dominant Polycystic Kidney Disease cases. These genes encode for proteins of the polycystin signaling complex, which regulates different signals, including 3’,5’-cyclic adenosine monophosphate (cAMP), mammalian target of rapamycin (mTOR), and epidermal growth factor receptor pathways.
There is currently no effective curative treatment for autosomal dominant polycystic kidney disease, and most efforts are aimed at mitigating the complications and progression or reducing cyst growth and delaying the eventual progression to kidney failure. JYNARQUE/JINARC/SAMSCA, developed by Otsuka Pharmaceuticals, is the only approved drug available globally for treating autosomal dominant polycystic kidney disease in adults. It inhibits vasopressin receptors in the kidneys and retards kidney function decline by reducing adenylate cyclase activity in adults at risk of rapidly progressing autosomal dominant polycystic kidney disease.
Nonetheless, being the only approved drug for the indication gives Otsuka an early mover advantage which is studying the effectiveness of Tolvaptan in pediatric subjects (28 days to less than 12 weeks old at the time of enrollment) with autosomal dominant polycystic kidney disease.
However, companies are conducting clinical trials that investigate new treatment options. Key players such as Regulus Therapeutic’s RGLS8429, Xortx Therapeutic’s XRx-008, Vertex Pharmaceutical’s VX-407, and others are investigating their candidates for the management of autosomal dominant polycystic kidney disease in the 7MM.
According to DelveInsight, the overall autosomal dominant polycystic kidney disease market dynamics are anticipated to change in the coming years owing to the expected launch of emerging therapies.
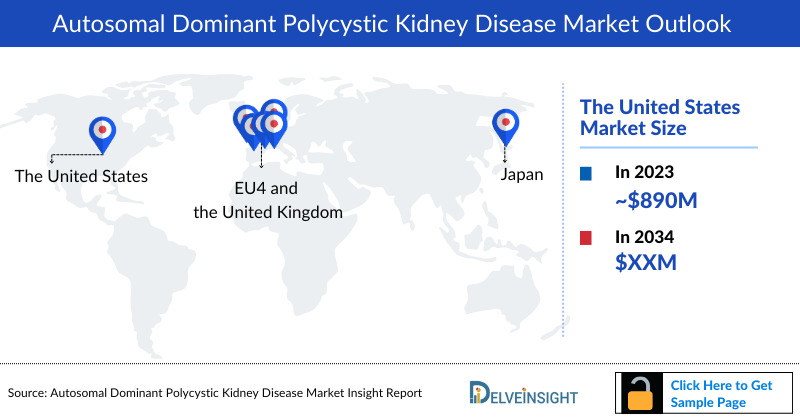
- In 2023, the Autosomal Dominant Polycystic Kidney Disease Treatment Market in the 7MM was approximately USD 1,476.9 million, out of which the US accounted for approximately USD 889.8 million. These numbers are expected to change with the launch of emerging therapies during the forecast period (2024-2034).
- DelveInsight’s analysts estimate that the Autosomal Dominant Polycystic Kidney Disease Drugs Market is expected to show positive growth, mainly attributed to the increased occurrence of kidney disorders, sedentary lifestyle leading to hypertension and obesity, and the launch of upcoming therapies during the forecast period (2024-2034).
- EU4 and the UK were nearly USD 7,395 million and accounted for nearly 39.4% of the total 7MM Autosomal Dominant Polycystic Kidney Disease Treatment Market Size in 2023.
- Among the EU4 and the UK, the UK holds the highest Autosomal Dominant Polycystic Kidney Disease Treatment Market Size of around USD 72.5 million followed by France, and Spain with approximately USD 65.6 million, and USD 29.4 million respectively. These numbers are expected to change by 2034.
- The Autosomal Dominant Polycystic Kidney Disease Treatment Market Size in Japan accounted for nearly 26% of the total 7MM Autosomal Dominant Polycystic Kidney Disease market size in 2023, these numbers are expected to change by 2034.
Autosomal Dominant Polycystic Kidney Disease Drugs Uptake
This section focuses on the uptake rate of potential Autosomal Dominant Polycystic Kidney Disease drugs expected to be launched in the market during 2020–2034. For example, RGLS8429, a novel, next-generation oligonucleotide for the treatment of autosomal dominant polycystic kidney disease designed to inhibit miR-17 and preferentially target the kidney is anticipated to enter the market during the forecast period, it is poised to revolutionize the market dynamics by offering a unique and effective treatment option for individuals with autosomal dominant polycystic kidney disease.
Autosomal Dominant Polycystic Kidney Disease Pipeline Development Activities
The Autosomal Dominant Polycystic Kidney Disease pipeline segment provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes Autosomal Dominant Polycystic Kidney Disease companies involved in developing targeted therapeutics.
Pipeline development activities
The Autosomal Dominant Polycystic Kidney Disease pipeline segment covers information on collaborations, acquisitions and mergers, licensing, and patent details for Autosomal Dominant Polycystic Kidney Disease emerging therapies.
KOL Views
To keep up with current Autosomal Dominant Polycystic Kidney Disease market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on autosomal dominant polycystic kidney disease evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Washington University School of Medicine, University of Cincinnati College of Medicine, University Hospital Freiburg, University Hospital of Cologne, Pediatrics Nephrology, Hôpital Necker-Enfants Malades, University of Sheffield Medical School, Medical Corporation Doyukai Kasuga Clinic, Kobe University Graduate School of Medicine were contacted.
Their opinion helps understand and validate current and emerging therapy treatment patterns or autosomal dominant polycystic kidney disease market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Autosomal Dominant Polycystic Kidney Disease unmet needs.
Physician’s View
According to our primary research analysis, despite considerable progress in autosomal dominant polycystic kidney disease treatment market, there persists a significant unmet need. One of the foremost challenges associated with autosomal dominant polycystic kidney disease treatment is the scarcity of disease-modifying treatments that can effectively halt or slow the progression.
While current therapies focus on symptom management and complication prevention, there is a critical need for interventions that address the underlying mechanisms of cyst formation and kidney damage. Physicians express concerns about the challenge of managing autosomal dominant polycystic kidney disease progression, particularly in patients with rapidly advancing disease or those who develop complications such as hypertension and kidney failure.
Furthermore, they have emphasized the importance of early intervention and the need for more effective treatment options to prevent or delay adverse outcomes. According to a KOL in the US, in patients with autosomal dominant polycystic kidney disease, long-term treatment with tolvaptan can cause severe and potentially life-threatening liver injury which is typically hepatocellular, and occurs between 3 and 18 months after starting tolvaptan, and resolves within 4 months after stopping the drug.
As per another KOL, treatments to slow disease progression in children with autosomal dominant polycystic kidney disease are limited, and no clear evidence exists to suggest that presymptomatic detection improves outcomes. In another KOL in Japan, it has been challenging to develop a pharmacotherapy for autosomal dominant polycystic kidney disease, an uncommon condition that leads to end-stage renal failure. For many years, the lack of curative therapy has been a major challenge for persons with autosomal dominant polycystic kidney disease. Samsca's clearance may be able to help these patients and their families in some way.
Autosomal Dominant Polycystic Kidney Disease Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Autosomal Dominant Polycystic Kidney Disease treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in autosomal dominant polycystic kidney disease trials, one of the most important primary outcome measures is complete eschar removal.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Autosomal Dominant Polycystic Kidney Disease Drugs Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the Autosomal Dominant Polycystic Kidney Disease drugs market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The reimbursement challenges related to medical care and treatment for individuals with autosomal dominant polycystic kidney disease can be significant as it often requires specialized medical attention, covering the costs of diagnosis, treatment, and ongoing care. Health insurance plans may not fully cover limited coverage of some medical treatments, and therapies specific to cervical dystonia.
This can result in high out-of-pocket expenses for families seeking the best care for their loved ones. Moreover, it requires specialized care from healthcare providers with expertise. Finding and accessing such specialists may be challenging, and the associated costs may not always be fully reimbursed by insurance.
Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Report Scope
- The Autosomal Dominant Polycystic Kidney Disease therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the Autosomal Dominant Polycystic Kidney Disease epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current Autosomal Dominant Polycystic Kidney Disease treatment market landscape.
- A detailed review of the autosomal dominant polycystic kidney disease drugs market, historical and forecasted Autosomal Dominant Polycystic Kidney Disease market size, Autosomal Dominant Polycystic Kidney Disease market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The patient-based Autosomal Dominant Polycystic Kidney Disease Market Forecasting report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM autosomal dominant polycystic kidney disease therapeutics market.
Autosomal Dominant Polycystic Kidney Disease Therapeutis Market Report Insights
- Patient-based Autosomal Dominant Polycystic Kidney Disease Market Forecasting
- Autosomal Dominant Polycystic Kidney Disease Therapeutic Approaches
- Autosomal Dominant Polycystic Kidney Disease Pipeline Drugs Analysis
- Autosomal Dominant Polycystic Kidney Disease Market Size
- Autosomal Dominant Polycystic Kidney Disease Market Trends
- Existing and Future Autosomal Dominant Polycystic Kidney Disease Drugs Market Opportunity
Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Report Key Strengths
- 11 years Autosomal Dominant Polycystic Kidney Disease Market Forecast
- The 7MM Coverage
- Autosomal Dominant Polycystic Kidney Disease Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Autosomal Dominant Polycystic Kidney Disease Drugs Uptake
- Key Autosomal Dominant Polycystic Kidney Disease Market Forecast Assumptions
Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Report Assessment
- Current Autosomal Dominant Polycystic Kidney Disease Treatment Market Practices
- Autosomal Dominant Polycystic Kidney Disease Unmet Needs
- Autosomal Dominant Polycystic Kidney Disease Pipeline Drugs Analysis Profiles
- Autosomal Dominant Polycystic Kidney Disease Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Autosomal Dominant Polycystic Kidney Disease Treatment Market Insights
- What was the autosomal dominant polycystic kidney disease drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What was the total Autosomal Dominant Polycystic Kidney Disease market size by therapies, and Autosomal Dominant Polycystic Kidney Disease market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will VX-407 and XRx-008 affect the treatment paradigm of autosomal dominant polycystic kidney disease?
- How will RGLS8429 compete with upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and Autosomal Dominant Polycystic Kidney Disease marketed therapies?
- How would future opportunities affect the Autosomal Dominant Polycystic Kidney Disease market dynamics and subsequent analysis of the associated trends?
Autosomal Dominant Polycystic Kidney Disease Epidemiology Insights
- What are the disease risks, burdens, and autosomal dominant polycystic kidney disease unmet needs?
- What will be the growth opportunities across the 7MM concerning the patient population with autosomal dominant polycystic kidney disease?
- What is the historical and forecasted autosomal dominant polycystic kidney disease patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the above-mentioned countries, which country would have the highest autosomal dominant polycystic kidney disease diagnosed prevalent population during the forecast period (2024–2034)?
- What factors are factors contributing to the growth of autosomal dominant polycystic kidney disease cases?
Current Autosomal Dominant Polycystic Kidney Disease Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Autosomal Dominant Polycystic Kidney Disease treatment?
- What are the current guidelines for treating autosomal dominant polycystic kidney disease in the US and Europe?
- How many Autosomal Dominant Polycystic Kidney Disease companies are developing therapies for the autosomal dominant polycystic kidney disease treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating autosomal dominant polycystic kidney disease?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing Autosomal Dominant Polycystic Kidney Disease therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of approved therapies?
- What is the 7MM historical and forecasted autosomal dominant polycystic kidney disease drugs market?
Reasons to Buy
- The patient-based Autosomal Dominant Polycystic Kidney Disease market forecasting report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the autosomal dominant polycystic kidney disease treatment market.
- Insights on patient burden Autosomal Dominant Polycystic Kidney Disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Autosomal Dominant Polycystic Kidney Disease treatment market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming Autosomal Dominant Polycystic Kidney Disease companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and Autosomal Dominant Polycystic Kidney Disease emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies for stuttering, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Autosomal Dominant Polycystic Kidney Disease unmet needs of the existing market so that the upcoming Autosomal Dominant Polycystic Kidney Disease companies can strengthen their development and launch strategy.
Access Exclusive Data Now! Click here to Read More about the Related Articles