Beta-thalassemia Market
DelveInsight's "Beta-thalassemia Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of the Beta-thalassemia, historical and forecasted epidemiology as well as the Beta-thalassemia market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
The Beta-thalassemia market report provides current treatment practices, emerging drugs, Beta-thalassemia market share of the individual therapies, current and forecasted Beta-thalassemia market Size from 2019 to 2032 segmented by seven major markets. The Report also covers current Beta-thalassemia treatment practice/algorithm, market drivers, market barriers and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the Beta-thalassemia market.
Geography Covered
- The United States
- EU5 (Germany, France, Italy, Spain, and the United Kingdom)
- Japan
Study Period: 2019-2032
Beta-thalassemia Disease Understanding and Treatment Algorithm
The DelveInsight’s Beta-thalassemia market report gives a thorough understanding of the Beta-thalassemia by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis, and treatment.
Beta-thalassemia Diagnosis
This segment of the report covers the detailed diagnostic methods or tests for Beta-thalassemia.
Beta-thalassemia Treatment
It covers the details of conventional and current medical therapies available in the Beta-thalassemia market for the treatment of the condition. It also provides Beta-thalassemia treatment algorithms and guidelines in the United States, Europe, and Japan.
Beta-thalassemia Epidemiology
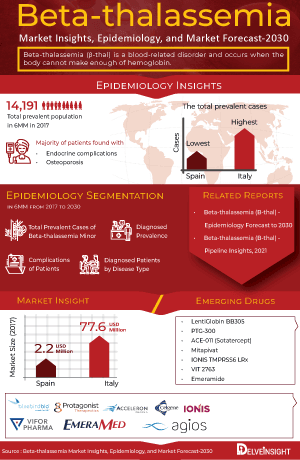
The Beta-thalassemia epidemiology section provides insights about the historical and current Beta-thalassemia patient pool and forecasted trends for individual seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the Beta-thalassemia market report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
The disease epidemiology covered in the report provides historical as well as forecasted Beta-thalassemia epidemiology scenario in the 7MM covering the United States, EU5 countries (Germany, Spain, Italy, France, and the United Kingdom), and Japan from 2019 to 2032.
Country Wise- Beta-thalassemia Epidemiology
The epidemiology segment also provides the Beta-thalassemia epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan.
Beta-thalassemia Drug Chapters
The drug chapter segment of the Beta-thalassemia report encloses the detailed analysis of Beta-thalassemia marketed drugs and late-stage (Phase-III and Phase-II) Beta-thalassemia pipeline drugs. It also helps to understand the Beta-thalassemia clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Beta-thalassemia Marketed Drugs
The report provides the details of the marketed products/off-label treatments available for Beta-thalassemia treatment.
Beta-thalassemia Emerging Drugs
The report provides the details of the emerging therapies under the late and mid-stage of development for Beta-thalassemia treatment.
Beta-thalassemia Market Outlook
The Beta-thalassemia market outlook of the report helps to build a detailed comprehension of the historic, current, and forecasted Beta-thalassemia market trends by analyzing the impact of current Beta-thalassemia therapies on the market, unmet needs, drivers and barriers, and demand for better technology.
This segment gives a thorough detail of Beta-thalassemia market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on the annual cost of therapy, inclusion and exclusion criteria's, mechanism of action, compliance rate, growing need of the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the market and view of the key opinion leaders. The calculated Beta-thalassemia market data are presented with relevant tables and graphs to give a clear view of the market at first sight.
According to DelveInsight, the Beta-thalassemia market in 7MM is expected to witness a major change in the study period 2019-2032.
Key Findings
This section includes a glimpse of the Beta-thalassemia market in 7MM.
The United States Market Outlook
This section provides the total Beta-thalassemia market size and market size by therapies in the United States.
EU-5 Countries: Market Outlook
The total Beta-thalassemia market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom is provided in this section.
Japan Market Outlook
The total Beta-thalassemia market size and market size by therapies in Japan is also mentioned.
Beta-thalassemia Drugs Uptake
This section focuses on the rate of uptake of the potential Beta-thalassemia drugs recently launched in the Beta-thalassemia market or expected to get launched in the market during the study period 2019-2032. The analysis covers Beta-thalassemia market uptake by drugs; patient uptake by therapies; and sales of each drug.
Beta-thalassemia Drugs Uptake helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allow the comparison of the drugs on the basis of Beta-thalassemia market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Beta-thalassemia Pipeline Development Activities
The Beta-thalassemia report provides insights into different therapeutic candidates in Phase II, and Phase III stage. It also analyses Beta-thalassemia key players involved in developing targeted therapeutics.
Pipeline Development Activities
The Beta-thalassemia report covers the detailed information of collaborations, acquisition, and merger, licensing, patent details, and other information for Beta-thalassemia emerging therapies.
Reimbursement Scenario in Beta-thalassemia
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we take reimbursement into consideration to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
KOL- Views
To keep up with current Beta-thalassemia market trends, we take KOLs and SMEs ' opinion working in the Beta-thalassemia domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Beta-thalassemia market trends. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the unmet needs.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Beta-thalassemia Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Report
- The report covers the descriptive overview of Beta-thalassemia, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
- Comprehensive insight has been provided into the Beta-thalassemia epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Beta-thalassemia is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Beta-thalassemia market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the global Beta-thalassemia market
Report Highlights
- In the coming years, the Beta-thalassemia market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Beta-thalassemia R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for Beta-thalassemia. The launch of emerging therapies will significantly impact the Beta-thalassemia market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Beta-thalassemia
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Beta-thalassemia Report Insights
- Beta-thalassemia Patient Population
- Therapeutic Approaches
- Beta-thalassemia Pipeline Analysis
- Beta-thalassemia Market Size and Trends
- Beta-thalassemia Market Opportunities
- Impact of upcoming Beta-thalassemia Therapies
Beta-thalassemia Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Beta-thalassemia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Drugs Uptake
Beta-thalassemia Report Assessment
- Current Treatment Practices
- Unmet Needs
- Beta-thalassemia Pipeline Product Profiles
- Beta-thalassemia Market Attractiveness
- Market Drivers and Barriers
Key Questions
Market Insights:
- What was the Beta-thalassemia drug class share (%) distribution in 2019 and how it would look like in 2032?
- What would be the Beta-thalassemia total market size as well as market size by therapies across the 7MM during the forecast period (2019-2032)?
- What are the key findings pertaining to the market across 7MM and which country will have the largest Beta-thalassemia market size during the forecast period (2019-2032)?
- At what CAGR, the Beta-thalassemia market is expected to grow by 7MM during the forecast period (2019-2032)?
- What would be the Beta-thalassemia market outlook across the 7MM during the forecast period (2019-2032)?
- What would be the Beta-thalassemia market growth till 2032, and what will be the resultant market Size in the year 2032?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights:
- What are the disease risk, burden, and regional/ethnic differences of the Beta-thalassemia?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What is the historical Beta-thalassemia patient pool in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What would be the forecasted patient pool of Beta-thalassemia in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- Where will be the growth opportunities in the 7MM with respect to the patient population pertaining to Beta-thalassemia?
- Out of all 7MM countries, which country would have the highest prevalent population of Beta-thalassemia during the forecast period (2019-2032)?
- At what CAGR the patient population is expected to grow in 7MM during the forecast period (2019-2032)?
Current Treatment Scenario, Marketed Drugs and Emerging Therapies:
- What are the current options for the Beta-thalassemia treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of Beta-thalassemia in the USA, Europe, and Japan?
- What are the Beta-thalassemia marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety and efficacy, etc.?
- How many companies are developing therapies for the treatment of Beta-thalassemia?
- How many therapies are in-development by each company for Beta-thalassemia treatment?
- How many are emerging therapies in mid-stage, and late stage of development for Beta-thalassemia treatment?
- What are the key collaborations (Industry - Industry, Industry - Academia), Mergers and acquisitions, licensing activities related to the Beta-thalassemia therapies?
- What are the recent novel therapies, targets, mechanisms of action and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Beta-thalassemia and their status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for Beta-thalassemia?
- What are the global historical and forecasted market of Beta-thalassemia?
Reasons to buy
- The report will help in developing business strategies by understanding trends shaping and driving the Beta-thalassemia market
- To understand the future market competition in the Beta-thalassemia market and Insightful review of the key market drivers and barriers
- Organize sales and marketing efforts by identifying the best opportunities for Beta-thalassemia in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom) and Japan
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for Beta-thalassemia market
- To understand the future market competition in the Beta-thalassemia market