Celiac Disease Market
- The Celiac Disease Market is poised for steady growth over the coming years as pharmaceutical companies invest in novel treatments aimed at improving the quality of life for patients living with this chronic autoimmune condition.
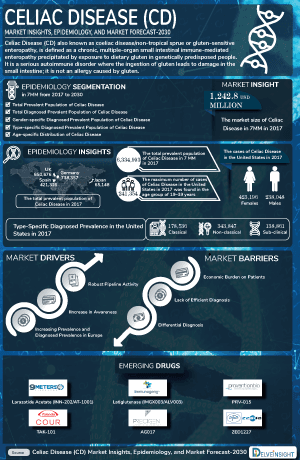
- Celiac Disease is an autoimmune disorder where ingestion of gluten triggers an immune response that damages the small intestine's lining.
- It affects approximately 1 in 100 people worldwide, yet only about 30% are properly diagnosed. The disease predominantly impacts females and is most commonly diagnosed in individuals aged 19-39, emphasizing the need for timely detection and management.
- The cornerstone of celiac disease management is a lifelong, strict gluten-free diet, which is essential for alleviating symptoms, promoting intestinal healing, and preventing long-term complications.
- For patients experiencing celiac crisis or refractory celiac disease type II (RCD II), short-term use of corticosteroids like prednisolone may provide relief until the gluten-free diet becomes effective. Additional treatment options for both RCD I and RCD II include Budesonide, systemic corticosteroids, 6-mercaptopurine, cladribine, mesalamine, mycophenolate mofetil, and methotrexate.
- Despite adherence to a gluten-free diet, approximately 5% of Celiac Disease Patients continue to suffer from symptoms, underscoring the urgent need for advanced treatment options and further research.
- In 2024, a landmark study identified that ZED1227, an investigational drug, successfully prevented gluten-induced intestinal damage by targeting specific molecular mechanisms, marking a significant step forward in celiac disease therapy.
- Recent progress in Celiac Disease Treatment was underscored by a July 2024 meeting where The Beyond Celiac Coalition—a collaborative alliance of multidisciplinary experts dedicated to advancing celiac disease therapies—engaged with the FDA. The meeting focused on adopting a patient-centric approach to therapeutic trials, aiming to enhance patient involvement while ensuring scientific rigor in the development of new treatments.
- The leading Celiac Disease Companies, such as Entero Therapeutics, Amgen/Provention Bio, Takeda, and Sanofi, are at the forefront of improving the treatment landscape and developing more effective therapies for celiac disease, promising new hope for better management and outcomes.
Request for Unlocking the Sample Page of the "Celiac Disease Treatment Market"
DelveInsight's “Celiac Disease Treatment Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of celiac disease, historical and forecasted epidemiology as well as the celiac disease market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Celiac Disease Treatment Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM celiac disease market size from 2020 to 2034. The report also covers current celiac disease treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Celiac Disease Epidemiology
|
Segmented by:
|
|
Celiac Disease Companies |
|
|
Celiac Disease Drugs |
|
|
Celiac Disease Therapeutics Market |
Segmented by:
|
|
Analysis |
|
Celiac Disease Treatment Market: Understanding and Algorithm
Celiac disease is a chronic autoimmune disorder driven by an inappropriate immune response to gluten, a protein in wheat, barley, and rye, in genetically predisposed individuals. The disease primarily targets the small intestine, causing inflammation and mucosal damage, leading to a spectrum of gastrointestinal symptoms like chronic Diarrhea, abdominal pain, and malabsorption, as well as extraintestinal issues such as anemia, osteoporosis, and neurological disturbances. The Celiac Disease Pathogenesis involves a complex interaction between genetic factors, particularly HLA-DQ2 and HLA-DQ8 haplotypes, and environmental triggers, including viral infections and gut microbiota alterations. These elements disrupt gluten tolerance, activating T cells and prompting the production of autoantibodies, primarily against tissue transglutaminase (tTG), a key autoantigen. Diagnosis, often complicated by varied clinical presentations, is typically confirmed through serological testing for specific antibodies and histological examination of intestinal biopsies.
The Celiac Disease Treatment Market Report provides an overview of celiac disease pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Celiac Disease Treatment
The primary treatment for celiac disease is a strict, lifelong gluten-free diet, which allows the small intestine to heal and alleviates symptoms. Following a gluten-free diet typically leads to symptom improvement within weeks to months. A registered dietitian can assist with meal planning, label reading, and making healthy food choices. In some cases, additional treatments such as vitamin or mineral supplements, or corticosteroids for persistent inflammation, may be needed. Regular follow-up with a healthcare provider is crucial to monitor progress and nutritional status. Despite strict adherence to the diet, some individuals may experience refractory celiac disease, requiring further evaluation and alternative treatments.
Celiac Disease Epidemiology
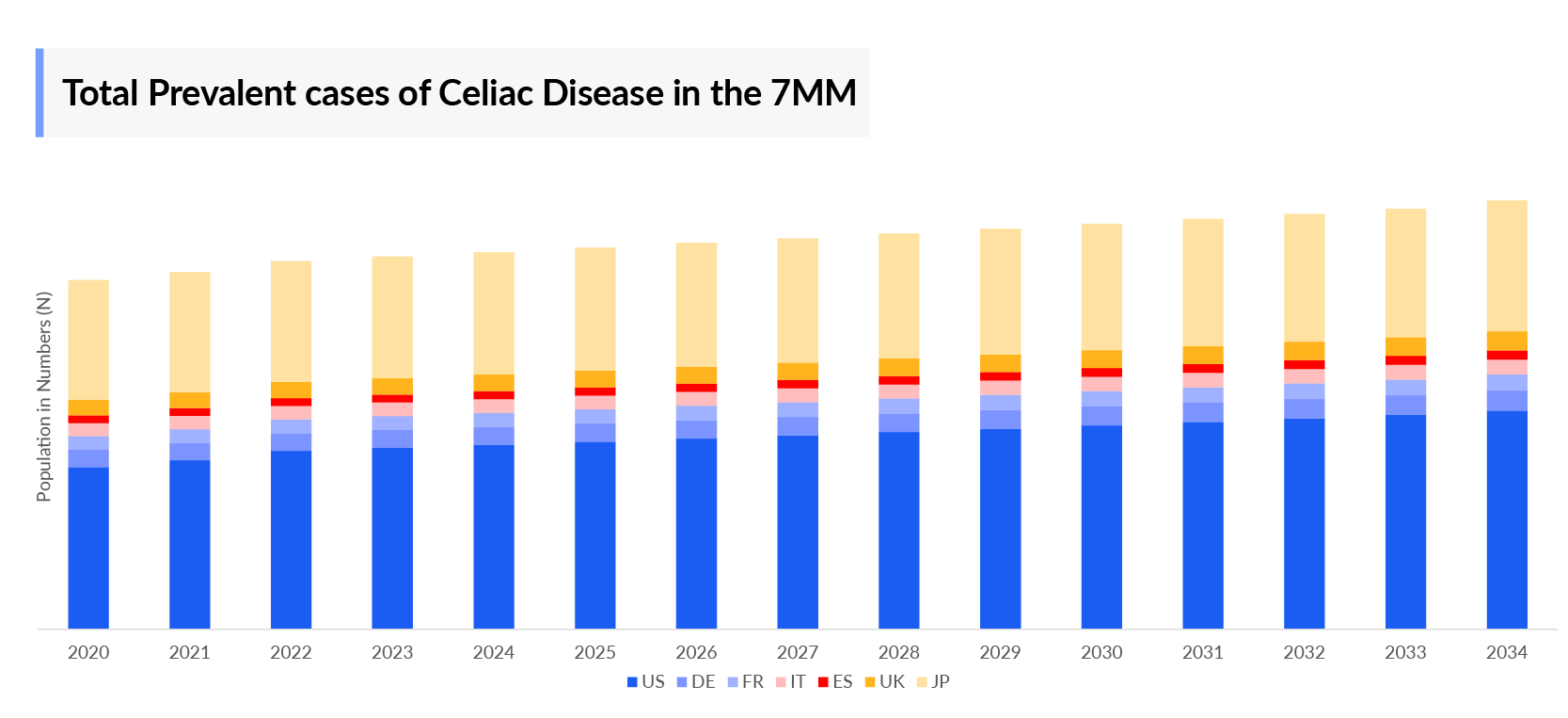
The celiac disease epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The celiac disease epidemiology is segmented with detailed insights into total Celiac Disease Prevalent Population, total Celiac Disease diagnosed prevalent population, Celiac Disease gender-specific diagnosed prevalent population, Celiac Disease type-specific diagnosed prevalent population, and Celiac Disease age-specific distribution.
- In 2023, the United States represented nearly 50% of the Celiac Disease Diagnosed Prevalent Cases across the 7MM
- Celiac disease shows a notable gender disparity, with females being disproportionately affected. For instance, in Germany, approximately 60% of diagnosed cases are female.
- In 2023, classical celiac disease comprised about 30% of all celiac disease cases in Japan.
Celiac Disease Recent Developments
- In May 2025, Teva Pharmaceutical announced FDA Fast Track designation for TEV-53408, an anti-IL-15 antibody in Phase 2a trials for treating adults with celiac disease on a gluten-free diet.
Celiac Disease Drugs Market Chapters
The drug chapter segment of the Celiac Disease Drugs Market Report encloses a detailed analysis of celiac disease marketed drugs and late-stage (Phase III and Phase II) Celiac Disease Pipeline Drugs analysis. It also deep dives into the Pivotal Celiac Disease Clinical Trials details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Celiac Disease Emerging Drugs
- Latiglutenase: Entero Therapeutics
Latiglutenase (IMGX003) is an orally administered mixture of two gluten-specific recombinant proteases that degrades gluten proteins into small physiologically irrelevant fragments that is designed to be administered as an adjunct to a gluten-free diet. The Phase II Celiac Disease Clinical Trials for latiglutenase have been successfully completed, and the Phase III development plan has been thoroughly reviewed by the Gastrointestinal Division of the US FDA.
- Ordesekimab: Amgen/ Provention Bio, a Sanofi Company
Ordesekimab (formerly AMG 714/PRV-015) is a monoclonal antibody that inhibits the action of Interleukin-15 (IL-15). It is being investigated for the treatment of non-responsive celiac disease as an adjunct to a gluten free diet. Ordesekimab is being developed in collaboration with Provention Bio, a Sanofi company. Currently, it is in Phase IIb of its Celiac Disease Clinical Trials development for the treatment of non responsive celiac disease.
|
Therapy Name |
Company Name |
ROA |
Celiac Disease MOA |
Any Special Status |
|
Latiglutenase |
Entero Therapeutics |
Oral |
Degrades gluten proteins |
FTD |
|
Ordesekimab |
Amgen/ Provention Bio |
Subcutaneous |
IL-15 Inhibitor |
NA |
|
TAK-101 |
Takeda |
Intravenous |
Initiate tolerogenic immune reprogramming |
FTD |
|
TAK-227 |
Takeda |
Oral |
Transglutaminase 2 inhibitors |
NA |
 Celiac Disease Market Outlook
Celiac Disease Market Outlook
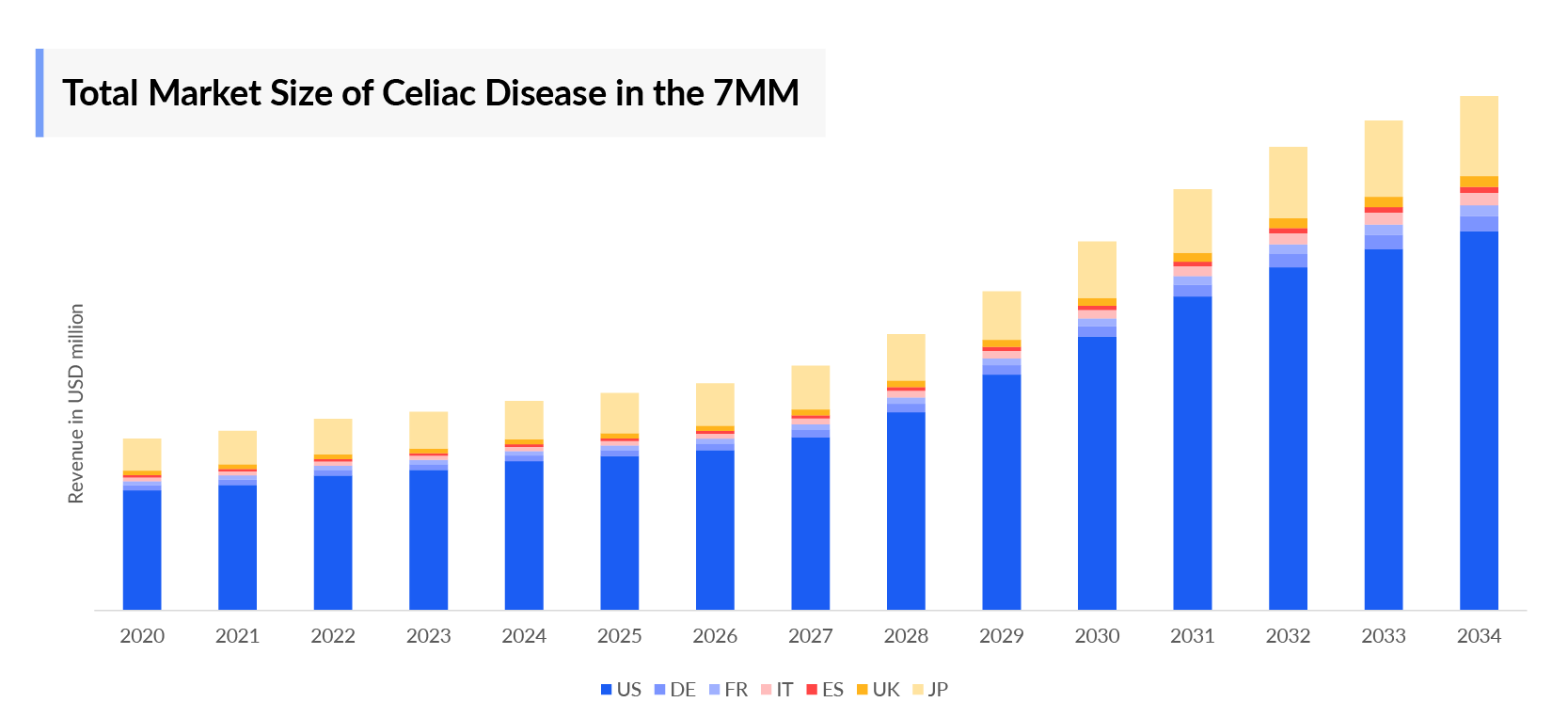
The Celiac Disease Market is set for notable growth, fueled by rising awareness, better diagnostics, and increasing Celiac Disease Prevalence. As diagnosis rates climb, demand for gluten-free products, targeted therapies, and advanced diagnostics is accelerating. The leading Celiac Disease Companies, including Entero Therapeutics, Amgen, Takeda, Sanofi, are actively developing innovative treatments to address unmet needs in this expanding Celiac Disease Drugs Market.
- In 2023, the United States held the largest share of the Celiac Disease Drugs Market within the 7MM, accounting for approximately 70% of the total market.
- Among the EU4 and the UK, Italy led with an estimated Celiac Disease Drugs Market valuation of approximately USD 200 million for celiac disease.
- Currently, gluten-free diet is the only effective Celiac Disease Treatment and in 2023, this market in Japan was valued at approximately USD 1,500 million.
Celiac Disease Drugs Uptake
This section focuses on the uptake rate of potential Celiac Disease drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with an order of entry. It is important to understand that the key Celiac Disease Companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake. Further detailed analysis of emerging therapies drug uptake in the report…
Celiac Disease Pipeline Development Activities
The Celiac Disease therapeutics market report provides insights into different therapeutic candidates in the Phase III and Phase II stages. It also analyzes key Celiac Disease Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Celiac Disease therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Celiac disease emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving Celiac Disease treatment market landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of celiac disease. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Celiac Disease drugs market and the unmet needs.
Celiac Disease Drugs Market: Qualitative Analysis
We perform Qualitative and Celiac Disease Drugs Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Celiac Disease Drugs Market Access and Reimbursement
The Celiac Disease Drugs Market Report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Celiac Disease Drugs Market Report Scope
- The Celiac Disease therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Celiac Disease treatment market landscape.
- A detailed review of the Celiac Disease treatment market, historical and forecasted Celiac Disease treatment market size, Celiac Disease drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Celiac Disease therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM celiac disease drugs market.
Celiac Disease Therapeutics Market Report Insights
- Patient-based Celiac Disease Market Forecasting
- Therapeutic Approaches
- Celiac disease Pipeline Drugs Analysis
- Celiac disease Treatment Market Size and Trends
- Existing and future Celiac Disease Diagnostics Market Opportunity
Celiac Disease Diagnostics Market Report Key Strengths
- 11 Years Celiac Disease Market Forecast
- 7MM Coverage
- Celiac Disease Epidemiology Segmentation
- Inclusion of Country specific Celiac Disease treatment guidelines
- KOL’s feedback on approved and Celiac Disease emerging therapies
- Key Cross Competition
- Celiac Disease Conjoint analysis
- Celiac Disease Drugs Uptake
- Key Celiac Disease Market Forecast Assumptions
Celiac Disease Diagnostics Market Report Assessment
- Current Celiac Disease Treatment Market Practices
- Celiac Disease Unmet Needs
- Celiac Disease Pipeline Drugs Profiles
- Celiac Disease Diagnostics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM celiac disease testing market?
- What was the celiac disease testing market size, the Celiac Disease testing market size by therapies, Celiac Disease diagnostics market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the Celiac Disease market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the Celiac Disease Treatment?
- How many companies are developing therapies for the Celiac Disease Treatment?
- What are the recent novel therapies, targets, Celiac Disease mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The Celiac Disease Testing Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the celiac disease Drugs Market.
- Insights on patient burden/disease Celiac Disease Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Celiac Disease Testing Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Celiac Disease Testing Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Celiac Disease Testing Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles