Cell and Gene Therapy in Parkinson’s Disease Market
- According to DelveInsight's Cell and Gene Therapy in Parkinson’s Disease Treatment Market report, the US accounted for the highest number of diagnosed population with Parkinson’s disease, with around 1.1 million cases, among the 7MM in 2023. These cases are attributed to increase during the forecast period (2024–2034).
- Epidemiological estimates show a predominance of Parkinson's disease cases among males in the 7MM, likely influenced by hormonal, genetic, and environmental factors, alongside potential differences in healthcare-seeking behavior and lifestyle preferences between genders.
- Parkinson's disease stages are categorized into five levels: Stage I (mild symptoms), Stage II (worsening symptoms but still independent), Stage III (moderate symptoms), Stage IV (severe symptoms, requiring assistance), and Stage V (advanced symptoms, often bedridden).
- The Cell and Gene Therapy in Parkinson’s Disease Market Size is poised for significant growth driven by numerous promising therapies in the pipeline. With innovative approaches targeting disease modification and symptom management, these advancements hold the potential to revolutionize treatment outcomes for Parkinson's patients, driving market expansion in the near future.
Request for unlocking the CAGR of the "Cell and Gene Therapy in Parkinson’s Disease Drug Market"
DelveInsight’s comprehensive report titled “Cell and Gene Therapy in Parkinson’s disease Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Cell and Gene Therapy in Parkinson’s disease. The report presents historical and projected epidemiological data covering Total Diagnosed Prevalent Cases of Parkinson’s Disease, Gender-specific Diagnosed Prevalent Cases of Parkinson’s Disease, Age-specific Diagnosed Prevalent Cases of Parkinson’s Disease, and Stage-specific Diagnosed Prevalent Cases of Parkinson’s Disease. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020-2034.
The report analyzes the existing treatment practices and unmet medical requirements in Cell and Gene Therapy in Parkinson’s disease. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Cell and Gene Therapy in Parkinson’s Disease Epidemiology |
|
|
Cell and Gene Therapy in Parkinson’s Disease Market |
|
|
Market Analysis |
|
|
Cell and Gene Therapy in Parkinson’s Disease Market Players |
|
|
Future opportunity |
The development of breakthrough cell and gene therapies, advancement in Gene-Editing technologies, increased awareness and access to care, addressing unmet medical needs and personalized medicine may open up new avenues for targeted treatments, providing further opportunities for market growth. |
Cell and Gene Therapy in Parkinson’s disease Overview
Parkinson’s disease is a progressive neurodegenerative disorder that is defined by the loss of dopaminergic neurons in the substantia nigra pars compacta (SN) located in the midbrain and associated with Lewy bodies. The cause of Cell and Gene Therapy in Parkinson’s disease is still unknown, but it is believed that exposure to environmental risk factors, along with an inherited susceptibility, maybe a possible cause. Although men are slightly more susceptible than women, Parkinson's disease typically affects people over the age of 60, but there are also cases where it occurs at an earlier age.
Parkinson's disease has both motor and non-motor symptoms. Motor symptoms include tremors, slowness of movement, rigidity, and postural instability. Non-motor symptoms may include cognitive impairment, mood disorders, sleep disturbances, and autonomic dysfunction. If left uncontrolled, these symptoms can significantly impact a patient's quality of life. Moreover, the progressive nature of the disease places a considerable burden on caregivers.
Cell and Gene Therapy in Parkinson’s disease Treatment Market
Diagnosis of Parkinson’s is primarily clinical, based on the presence of characteristic motor and non-motor symptoms. Furthermore, neuroimaging, such as dopamine transporter (DAT) scans, magnetic resonance imaging (MRI), positron emission tomography (PET), DaTSCAN-SPECT, and others, may support clinical evaluation.
Cell and Gene Therapy in Parkinson’s disease is a condition that affects movement and is currently incurable. There are several medications available to manage the symptoms of the disease, but none of them can reverse its effects. These medications include levodopa, dopamine agonists, MAO-B inhibitors, COMT inhibitors, amantadine, and anticholinergics. The primary goal of these medications is to reduce the motor symptoms that people with Parkinson's disease experience. However, these treatments are only symptomatic and do not prevent the progression of the disease. Additionally, they are often associated with significant side effects.
Cell and Gene Therapy in Parkinson’s disease Epidemiology
The epidemiology section on the Cell and Gene Therapy in Parkinson’s disease market report offers information on the patient populations, including historical and projected trends for each of the 7MM. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of Cell and Gene Therapy in Parkinson’s disease. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Cell and Gene Therapy in Parkinson’s disease Key Findings
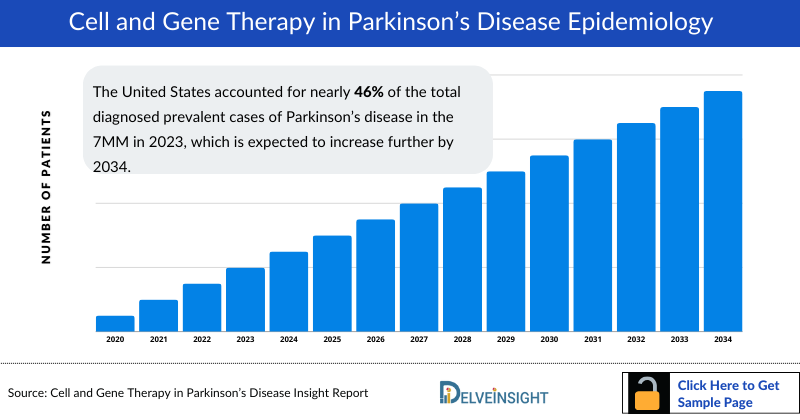
- The United States accounted for nearly 46% of the total diagnosed prevalent cases of Parkinson's disease in the 7MM in 2023, which is expected to increase further by 2034.
- As per the insights, the gender distribution of the disease suggests a male predominance, with approximately 54% male cases and 46% female cases in the 7MM in 2023.
- The age-specific distribution of the disease suggests that the age cohort of ≥75 years made the majority of the cases (64%), followed by 65–74 years (22%) in EU4 and the UK in 2023. While there were fewer cases in the age group ≤49 years in EU4 and the UK.
- In 2023, among the 7MM, Japan had the fourth-highest diagnosed prevalent cases, representing around 8% of the total diagnosed cases of Parkinson's disease in the 7MM.
- In 2023, the highest diagnosed prevalent cases of Parkinson’s disease were found in Stage III, i.e., around about 1 million cases, followed by Stage IV in the 7MM. An increasing trend is observed throughout the study period (2020–2034).
Cell and Gene Therapy in Parkinson’s Disease Market Outlook
Current Cell and Gene Therapy in Parkinson’s disease market relieve symptoms by replacing lost dopamine through medication, disease management, and lifestyle tools, such as exercise and complementary therapies. Different companies are pursuing the potential behind various investigational gene therapies to regrow or replace dopamine or rescue dying cells.
Cell-based strategies focusing on the replacement or protection of dopaminergic neurons have also been considered as a potential approach to treat Parkinson’s disease. Several preclinical studies using animal models have also suggested the potential benefits of mesenchymal stem cells (MSCs) for Parkinson's disease.
The current emerging pipeline for cell and gene therapies in Parkinson’s is robust with various therapies being developed like MeiraGTx’s AAV-GAD, Hope Biosciences’ HB-adMSCs, Sumitomo Pharma’s CT1-DAP001/DSP-1083, Prevail Therapeutics’ (Eli Lilly) PR001 (LY3884961), BlueRock Therapeutics’ Bemdaneprocel (BRT-DA01), and others.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Cell and Gene Therapy in Parkinson ’s disease market in the 7MM is projected to grow significantly during the study period 2020–2034.
Cell and Gene Therapy in Parkinson ’s disease Drug Chapters
Emerging Cell and Gene Therapy in Parkinson’s Disease Drugs
Prevail Therapeutics (Eli Lilly): PR001 (LY3884961)
PR001 is a Cell and Gene Therapy in Parkinson’s disease and neuronopathic Gaucher disease. It will be delivered through intra-cisterna magna injection. The GBA1 gene produces the lysosomal enzyme beta-glucocerebrosidase (GCase), which disposes and recycles cellular components. Patients with Parkinson's disease have mutations in their chromosomal copy of GBA1. The PROPEL clinical trial is evaluating the safety of LY3884961 administration in patients with moderate to severe Parkinson's disease with at least one pathogenic GBA1 mutation. The US FDA has granted fast-track designation for PR001 for the treatment of Parkinson’s disease with GBA1 mutations.
MeiraGTx: AAV-GAD
AAV-GAD is an investigational Cell and Gene Therapy in Parkinson’s disease treatment market, aiming to enhance GABA production in the brain's subthalamic nucleus. MeiraGTx acquired the therapy from Vector Neurosciences and is currently conducting Phase I/II trials to assess safety and tolerability. Global regulatory discussions for pivotal trial design are ongoing, with a potential pivotal study in 2024. Notably, AAV-GAD is the first gene therapy for PD with an imaging biomarker correlating with clinical improvement.
Hope Biosciences: HB-adMSCs
Hope Biosciences' HB-AdMSCs are adipose-derived mesenchymal stem cells processed through their proprietary cell culture platform. These pure mesenchymal stem cells, sourced from adult fat, are being developed as cell-based therapeutics for various conditions. Currently, Hope Bio is culturing cells for a Phase II clinical trial authorized by the US FDA and conducted by Hope Biosciences Stem Cell Research Foundation (HBSCRF). This randomized study aims to evaluate the efficacy and safety of multiple intravenous infusions of autologous adipose-derived mesenchymal stem cells in improving activities of daily living and quality of life in individuals with Parkinson’s Disease.
Note: Detailed emerging therapies assessment will be provided in the final report.
Cell and Gene Therapy in Parkinson’s Disease Market Segmentation
DelveInsight’s ‘Cell and Gene Therapy in Parkinson’s Disease Market Size, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Cell and Gene Therapy in Parkinson’s Disease market, segmented within countries and by therapies. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Cell and Gene Therapy in Parkinson’s Disease Market Size by Countries
The Cell and Gene Therapy in Parkinson’s Disease Market Size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2024, the United States held a significant share of the overall 7MM (Seven Major Markets) Cell and Gene Therapy in Parkinson’s Disease market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Cell and Gene Therapy in Parkinson’s Disease Market Size by Therapies
Cell and Gene Therapy in Parkinson’s Disease Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. Some of the key market players in the emerging pipeline are Prevail Therapeutics, MeiraGTx, Hope Biosciences, and others.
Note: Detailed market segment assessment will be provided in the final report..
Cell and Gene Therapy in Parkinson’s Disease Drugs Uptake
This section focuses on the sales uptake of potential Cell and Gene Therapy in Parkinson’s Disease drugs that have recently been launched or are anticipated to be launched in the Cell and Gene Therapy in Parkinson’s Disease market between 2020 and 2034. It estimates the market penetration of Cell and Gene Therapy in Parkinson’s Disease drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Cell and Gene Therapy in Parkinson’s Disease market.
Note: Detailed assessment of drug uptake will be provided in the full report on Cell and Gene Therapy in Parkinson’s Disease.
Cell and Gene Therapy in Parkinson’s Disease Market Access and Reimbursement
DelveInsight’s ‘Cell and Gene Therapy in Parkinson’s Disease – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Cell and Gene Therapy in Parkinson’s Disease.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Cell and Gene Therapy in Parkinson’s Disease market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Cell and Gene Therapy in Parkinson’s Disease domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Cell and Gene Therapy in Parkinson’s Disease market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market of Cell and Gene Therapy in Parkinson’s Disease unmet needs.
Cell and Gene Therapy in Parkinson’s Disease: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as Parkinson’s Foundation, Miami, US, The Cambridge Institute for Medical Research, The Keith Peters Building, Cambridge Biomedical Campus, Hills Road, CB2 0XY Cambridge, UK, Department of Clinical Neurosciences, University of Cambridge, Cambridge Biomedical Campus, Hills Road, CB2 0QQ Cambridge, UK among others.
“An annual occurrence of Parkinson’s disease among adults aged 65 and older is 50% higher than previous estimates of 60,000 diagnoses annually, thus making Parkinson’s disease the second most common age-related neurodegenerative condition diagnosed in North America.”
Note: Detailed assessment of KOL Views will be provided in the full report on Cell and Gene Therapy in Parkinson’s Disease.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Cell and Gene Therapy in Parkinson’s Disease Market, utilizing various Competitive Intelligence tools such as SWOT analysis, Conjoint Analysis, and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
The emerging Cell and Gene Therapy in Parkinson’s Disease therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Cell and Gene Therapy in Parkinson’s Disease market.
Note: Detailed assessment of SWOT analysis and Conjoint analysis will be provided in the full report on Cell and Gene Therapy in Parkinson’s Disease.
Cell and Gene Therapy in Parkinson’s Disease Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for Cell and Gene Therapy in Parkinson’s Disease. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Cell and Gene Therapy in Parkinson’s Disease Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Cell and Gene Therapy in Parkinson’s Disease.
Cell and Gene Therapy in Parkinson’s Disease Report Insights
- Parkinson’s Disease Patient Population
- Therapeutic Approaches
- Cell and Gene Therapy in Parkinson’s Disease Pipeline Analysis
- Cell and Gene Therapy in Parkinson’s Disease Market Size and Trends
- Cell and Gene Therapy in Parkinson’s Disease Market Opportunities
- Impact of Upcoming Therapies
Cell and Gene Therapy in Parkinson’s Disease Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Parkinson’s Disease Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Cell and Gene Therapy in Parkinson’s Disease Market
- Cell and Gene Therapy in Parkinson’s Disease Drugs Uptake
Cell and Gene Therapy in Parkinson’s Disease Report Assessment
- Cell and Gene Therapy in Parkinson’s Disease Current Treatment Practices
- Unmet Needs
- Cell and Gene Therapy in Parkinson’s Disease Pipeline Product Profiles
- Cell and Gene Therapy in Parkinson’s Disease Market Attractiveness
Cell and Gene Therapy in Parkinson’s Disease Key Questions
- How common is Cell and Gene Therapy in Parkinson’s disease?
- What are the key findings in Parkinson’s disease epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available Cell and Gene Therapy in Parkinson’s disease?
- What is the disease risk, burden, and unmet needs of Cell and Gene Therapy in Parkinson’s disease?
- At what CAGR is the Cell and Gene Therapy market in Parkinson’s disease is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the market of Cell and Gene Therapy in Parkinson’s disease market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool for Cell and Gene Therapy in Parkinson’s Disease in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many companies are currently developing Cell and Gene therapies for the treatment of Parkinson’s disease?
Ready to Dive Deeper? Purchase the Complete Report for in-depth Market Analysis by Clicking Here @ Cell and Gene Therapy in Parkinson’s Disease Market Forecast
Access Exclusive Data Now! Click here to Read More about the Related Articles @ Latest DelveInsight Blog