Charcot-Marie-Tooth Disease Market Summary
- The Charcot-Marie-Tooth Disease Market Size is anticipated to grow with a significant CAGR during the study period (2020-2034).
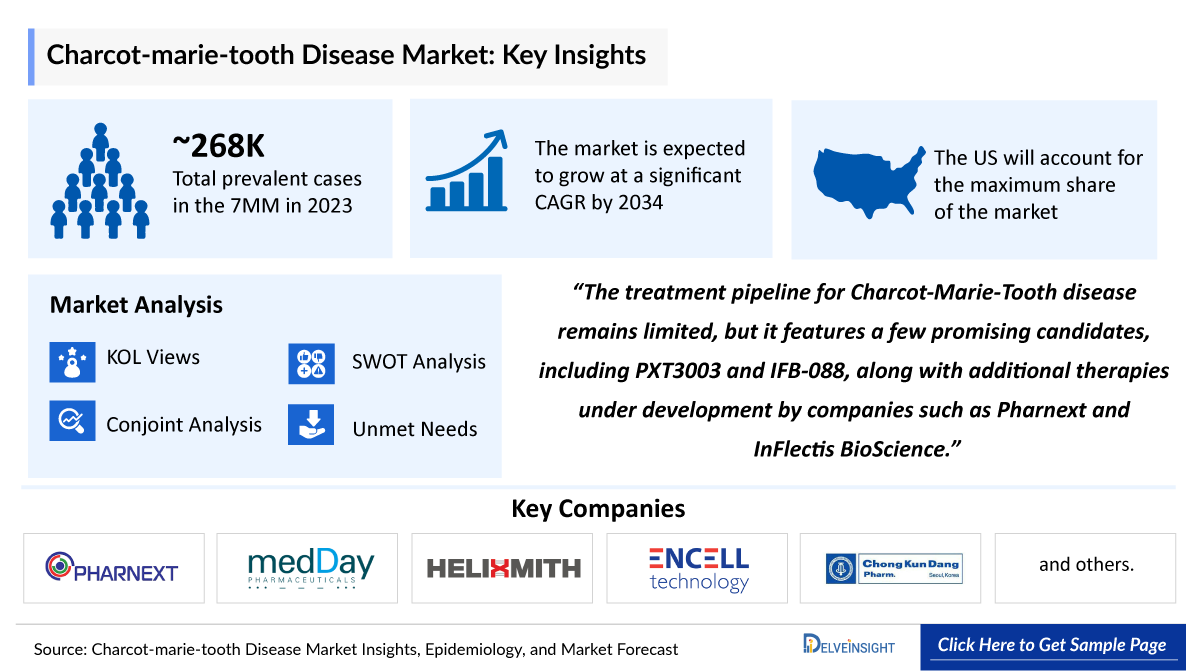
- The key Charcot-Marie-Tooth Disease companies developing therapies include - Pharnext, MedDay Pharmaceuticals, HELIXMITH, ENCell, Chong Kun Dang pharmaceutical, InFlectis BioScience, Addex Therapeutics, Augustine therapeutics, DTx Pharma, and others.
Charcot-Marie-Tooth Disease Market and Epidemiology Analysis
- The United States dominates the market for Charcot-Marie-Tooth disease, surpassing the EU4 (Germany, Spain, Italy, and France), the UK, and Japan in market size. In the seven major markets (7MM), the total market for Charcot-Marie-Tooth disease therapies is significantly influenced by non-opioid analgesics, which accounted for 43% of the 2023 US market share.
- Charcot-Marie-Tooth disease, also known as hereditary motor and sensory neuropathies, is one of the most commonly inherited neuromuscular disorders. It is a nerve-length-dependent condition marked by gradually worsening foot deformities, sensory loss, lower limb weakness, and diminished or absent deep tendon reflexes.
- In 2023, there were approximately 268,000 prevalent cases of Charcot-Marie-Tooth Disease across the seven major markets (7MM), with about 70% of cases diagnosed. This increase in diagnosed prevalent cases is attributed to improved diagnostic methods, heightened awareness, advancements in genetic testing, and the hereditary nature of the condition.
- According to 2023 estimates, approximately 55% of Charcot-Marie-Tooth disease cases were reported in males. This may be due to X-linked forms of the disease, which disproportionately affect males, as well as potential sex-based differences in the expression of certain genetic mutations associated with Charcot-Marie-Tooth disease.
- Currently, there are no FDA-approved drugs for the treatment of Charcot-Marie-Tooth disease, highlighting a significant unmet need. The lack of approval is attributed to the complexity of the disease, the variability in genetic mutations among patients, and the challenges in demonstrating clinical efficacy and safety in trials.
- The pipeline for treating Charcot-Marie-Tooth disease is not extensive, but it includes several promising candidates such as PXT3003 and IFB-088, along with other drugs currently in development by companies like Pharnext and InFlectis BioScience.
Request for unlocking the Sample Page of the "Charcot-Marie-Tooth Disease Market Insights"
Key Factors Driving the Growth of the Charcot-Marie-Tooth Disease Market
- Growing Prevalence and Awareness: Charcot-Marie-Tooth Disease is among the most common inherited neurological disorders. Rising awareness campaigns and improved genetic testing are leading to better identification of patients worldwide.
- Advances in Genetic Testing and Diagnostics: Next-generation sequencing and advanced diagnostic tools are making it easier to detect Charcot-Marie-Tooth Disease subtypes, enabling personalized treatment approaches and expanding the diagnosed patient pool.
- Pipeline of Emerging Therapies: Pharmaceutical and biotech companies are actively developing novel gene therapies, small molecules, and biologics to address disease progression, driving hope and market expansion.
- Government and Regulatory Support: Orphan drug incentives, rare disease funding, and regulatory fast-track designations are encouraging innovation and investment in Charcot-Marie Tooth Disease treatment research.
- Rising Patient Advocacy and Research Collaborations: Patient organizations and foundations are driving clinical trial participation, awareness, and funding, fostering collaborations between industry, academia, and healthcare providers to accelerate progress.
DelveInsight's “Charcot-Marie Tooth Disease Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of the indication of Charcot-Marie-Tooth Disease, historical and forecasted epidemiology as well as the Charcot-Marie-Tooth Disease therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The Charcot-Marie-Tooth Disease market report provides real-world prescription pattern analysis, approved drugs, market share of individual therapies, and historical and forecasted 7MM Charcot-Marie-Tooth Disease market size from 2020 to 2034. The report also covers current Charcot-Marie-Tooth Disease treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Charcot-Marie-Tooth Disease Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Charcot-Marie Tooth Disease Market |
|
|
Charcot-Marie Tooth Diseases Market Size | |
|
Charcot-Marie Tooth Disease Companies |
Pharnext, MedDay Pharmaceuticals, HELIXMITH, ENCell, Chong Kun Dang pharmaceutical, InFlectis BioScience, Addex Therapeutics, Augustine therapeutics, DTx Pharma, and others |
|
Charcot-Marie Tooth Disease Epidemiology Segmentation |
|
Charcot-Marie-Tooth Disease understanding
Charcot-Marie-Tooth Disease Overview
Charcot-Marie-Tooth disease (CMT), also known as hereditary motor and sensory neuropathies, is the most common inherited neuromuscular disorder. It affects the peripheral nerves, leading to progressive degeneration that primarily impacts the long nerves extending to the feet and hands. Charcot-Marie-Tooth disease is characterized by muscle weakness, sensory loss, foot deformities such as high arches (pes cavus), and reduced or absent deep tendon reflexes.
Charcot-Marie-Tooth Disease Diagnosis
The many different types of CMT are distinguished by the age of onset, inheritance pattern, severity, and whether they are linked to defects in axons or myelin: CMT1, which involves damage to the myelin sheath, and CMT2, which affects the axons directly. Diagnosis typically involves a clinical evaluation, genetic testing to identify specific mutations, and nerve conduction studies to differentiate between the demyelinating and axonal types of the disease. Charcot-Marie-Tooth disease is a slowly progressive condition, with symptoms varying in severity even within the same family.
The Charcot-Marie-Tooth disease report provides an overview of Charcot-Marie-Tooth disease pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Charcot-Marie-Tooth Disease Treatment
Management of Charcot-Marie-Tooth disease is primarily supportive and can significantly enhance a patient’s quality of life. With no cure available, treatment aims to manage symptoms and preserve mobility and function, focusing on relieving pain, strengthening muscles, and addressing functional impairments.
- Physical Therapy: A cornerstone of CMT management, physical therapy focuses on strengthening muscles, improving balance, and maintaining mobility. Regular exercises can help slow the progression of muscle weakness and prevent joint deformities.
- Orthotic Devices: Many patients benefit from custom orthotics, such as ankle-foot orthoses (AFOs), which provide stability, prevent foot drop, and improve walking ability. In severe cases, wheelchairs or other mobility aids may be necessary.
- Pain Management: Pain associated with CMT can be managed through medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), gabapentin, or pregabalin. In some cases, opioids or other pain management strategies may be considered.
- Surgical Interventions: For some patients, surgery may be necessary to correct severe foot deformities, improve function, or relieve pain. Common procedures include tendon transfers, osteotomies, or joint fusions.
Charcot-Marie-Tooth Disease Epidemiology
The Charcot-Marie Tooth disease epidemiology chapter in the report provides historical as well as forecasted prevalence in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Charcot-Marie-Tooth Disease epidemiology is segmented with detailed insights into Total Prevalent Cases of Charcot–Marie–Tooth, Diagnosed Prevalent Cases of Charcot–Marie–Tooth, Gender-specific Diagnosed Prevalent Cases of Charcot–Marie–Tooth, Type-specific Diagnosed Prevalent cases of Charcot–Marie–Tooth, Age-specific Diagnosed Prevalent Cases of Charcot–Marie–Tooth in the 7MM [2020-2034].
Charcot-Marie-Tooth Disease Epidemiology Key Findings
- As per DelveInsight's estimates, in the year 2023, the total prevalent cases of Charcot-Marie-Tooth Disease were 268,000 cases in the 7MM, which might rise by 2034 at a CAGR of XX%.
- In the United States, only 70% of patients receive a diagnosis within a year due to several factors, including the variability of symptoms, lack of awareness, limited access to specialized genetic testing, and the complexity of distinguishing Charcot-Marie-Tooth disease from other neuropathies.
- In 2023, Charcot-Marie-Tooth disease type 1 (CMT1) was the most common form among the various types of CMT in the United States. This prevalence is largely due to the genetic mutations associated with CMT1, particularly those affecting the PMP-22 gene, and CMT1A, was the most prevalent subtype.
- EU4 and the UK accounted for approximately 42% of the total diagnosed prevalent cases of Charcot-Marie-Tooth Disease in the year 2023.
- According to the 2023 estimates, 55% of Charcot-Marie-Tooth disease cases were reported in males. This higher prevalence may be due to X-linked forms of the disease, which disproportionately affect males.
Charcot-Marie-Tooth Disease Epidemiology Segmentation
- Total Prevalent Cases of Charcot Marie Tooth in the 7MM
- Diagnosed Prevalent Cases of Charcot Marie Tooth in the 7MM
- Gender-specific Diagnosed Prevalent Cases of Charcot Marie Tooth in the 7MM
- Type-specific Diagnosed Prevalent cases of Charcot Marie Tooth in the 7MM
- Age-specific Diagnosed Prevalent Cases of Charcot Marie Tooth in the 7MM
Recent Developments In The Charcot-Marie-Tooth Disease Treatment Market
- In March 2025, ENCell, a leading biopharmaceutical company specializing in cell and gene therapy CDMO and novel drug development, announced that its investigational drug EN001 has received Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) for the treatment of Charcot-Marie-Tooth disease (CMT).
- In January 2025, NMD Pharma A/S announced that the U.S. FDA granted orphan drug designation (ODD) for NMD670, a novel oral small molecule inhibitor of the skeletal muscle-specific chloride ion channel ClC-1, for the treatment of Charcot-Marie-Tooth disease (CMT).
Charcot-Marie-Tooth Disease Drug Analysis
The drug chapter segment of the Charcot-Marie-Tooth Disease report encloses a detailed analysis of Charcot-Marie-Tooth Disease marketed drugs. It also deep dives into the Charcot-Marie-Tooth Disease pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Charcot-Marie Tooth Disease Emerging Drugs
PXT3003: Pharnext
PXT3003 is a novel, synergistic, low-dose fixed combination of baclofen (a muscle relaxant), naltrexone (an opioid antagonist), and sorbitol (a laxative) given twice daily as an oral solution. The product has multiple mechanisms of action: inhibition of the overexpression of the PMP22 gene associated with an improvement in myelination, preservation of the peripheral nerve axon, and additional beneficial effects on other cell types: muscle cells, neuromuscular junctions, and immune cells.
In April 2024, Pharnext presented the latest data from its ongoing analysis of the pivotal Phase III PREMIER trial evaluating PXT3003 for the treatment of Charcot-Marie-Tooth disease type 1A (CMT1A).
IFB-088: InFlectis BioScience
IFB-088 is a first-in-class orally available small molecule drug candidate with a validated mechanism of action and a promising pharmacokinetic profile capable of crossing the blood-brain barrier to target the central and peripheral nervous system. IFB-088 is a multifunctional drug that modulates the PPP1R15A/PP1c phosphatase complex and inhibits NR2B-containing NMDAR.
The Company is now planning a Phase II clinical trial in the United States and Europe.
Charcot-Marie Tooth Disease Emerging Drugs | ||||||
|
Drug Name |
Company |
Indications |
Phase |
RoA |
MoA |
Designations |
|
PXT3003 |
Pharnext |
Charcot-Marie-Tooth disease type 1A |
III |
Oral |
Modulates the expression of the PMP22 gene |
FTD |
|
IFB-088 |
InFlectis BioScience |
Charcot-Marie-Tooth disease |
II |
Oral |
Modulates the splicing of the PMP-22 gene |
NA |
Note: Detailed emerging therapies assessment will be provided in the full report of Charcot-Marie-Tooth Disease...
Charcot-Marie-Tooth Disease Market Outlook
The market size for Charcot-Marie-Tooth Disease in the seven major markets is projected to experience significant growth from 2024, driven by a notable increase in prevalent cases and the necessity for innovative therapies that can provide more effective, long-term relief for the millions of women affected by Charcot-Marie-Tooth Disease.
- The United States accounts for the largest market size of Charcot-Marie-Tooth Disease, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Among EU4 and the UK, Germany had the largest market size accounting for approximately USD 4 million, followed by France, and the UK in 2023.
- In Japan, the total market size of Charcot-Marie-Tooth Disease was USD 2 million in 2023, which is expected to rise during the study period (2020–2034).
- In 2023, non-opioid analgesics dominated 43% of the Charcot-Marie-Tooth Disease market, while prescription medications, particularly opioid analgesics, and antidepressants along with post-surgical medications, held the remaining 57%.
Charcot Marie Tooth Disease Competitive Landscape
The competitive landscape for the Charcot Marie Tooth disease market is evolving rapidly as the field transitions from predominantly supportive care toward disease-modifying therapies. Historically, CMT management focused on physical therapy, orthopedic devices, and symptom control, with limited pharmacological options. However, increased research activity and a growing number of companies developing targeted treatments are reshaping competition.
Key players include biotech and specialty pharma firms advancing innovative candidates aimed at modifying disease progression rather than only managing symptoms. Multiple companies such as those developing gene-targeted therapies, small molecules, and novel mechanisms are actively conducting clinical trials, indicating a diversifying pipeline with significant future potential. The competitive field also benefits from strong involvement by research foundations and collaborations that accelerate development of next-generation therapies.
Overall, competition is driven by scientific innovation, genetic target validation, and regulatory incentives for rare diseases, with firms positioning themselves through clinical advancements and strategic partnerships to capture emerging opportunities in the expanding CMT therapeutic market.
Key Charcot Marie Tooth Disease Companies
The key companies involved in the Charcot-Marie-Tooth disease market including those advancing pipeline therapies and emerging treatment approaches:
- Pharnext
- MedDay Pharmaceuticals
- HELIXMITH
- ENCell
- Chong Kun Dang pharmaceutical
- InFlectis BioScience
- Addex Therapeutics
- Augustine therapeutics
- DTx Pharma, and others
Charcot-Marie-Tooth Disease Drug Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Charcot-Marie-Tooth Disease Clinical Trials Activities
The Charcot-Marie Tooth Disease pipeline report provides insights into different Charcot-Marie Tooth Disease clinical trails. It also analyzes key players involved in developing targeted therapeutics.
Charcot-Marie Tooth Disease Pipeline Development Activities
The Charcot-Marie Tooth Disease clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies.
Latest KOL Views on Charcot-Marie Tooth Disease
To keep up with the real-world scenario in current market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current treatment patterns of Charcot-Marie-Tooth Disease. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Charcot-Marie Tooth Disease Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy.
Charcot-Marie Tooth Disease Market Access and Reimbursement
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Charcot-Marie-Tooth Disease Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Charcot-Marie-Tooth Disease, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country-specific treatment guidelines.
- Additionally, an all-inclusive account of the current therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Charcot-Marie-Tooth Disease market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Charcot-Marie-Tooth Disease.
Charcot-Marie-Tooth Disease Market Report Insights
- Charcot-Marie-Tooth Disease Patient Population
- Charcot-Marie-Tooth Disease Therapeutic Approaches
- Charcot-Marie-Tooth Disease Pipeline Analysis
- Charcot-Marie-Tooth Disease Market Size and Trends
- Existing and future Market Opportunity
Charcot-Marie-Tooth Disease Market Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Charcot-Marie-Tooth Disease Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved therapies
- Key Cross Competition
- Conjoint analysis
- Charcot-Marie-Tooth Disease Drugs Uptake
- Key Charcot-Marie-Tooth Disease Market Forecast Assumptions
Charcot-Marie-Tooth Disease Market Report Assessment
- Current Charcot-Marie-Tooth Disease Treatment Practices
- Charcot-Marie-Tooth Disease Unmet Needs
- Charcot-Marie-Tooth Disease Pipeline Product Profiles
- Charcot-Marie-Tooth Disease Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Charcot-Marie-Tooth Disease Market Drivers
- Charcot-Marie-Tooth Disease Market Barriers
FAQs Realted to the Charcot-Marie-Tooth Disease Market Disease
- What is the growth rate of the 7MM Charcot-Marie-Tooth Disease treatment market?
- What was the Charcot-Marie-Tooth Disease total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current options for the treatment of Charcot-Marie-Tooth Disease ?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Charcot-Marie-Tooth Disease Market Forecast Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Charcot-Marie-Tooth Disease Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Detailed analysis and ranking of class-wise potential current therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.