Chronic Obstructive Pulmonary Disease (COPD) Market Summary
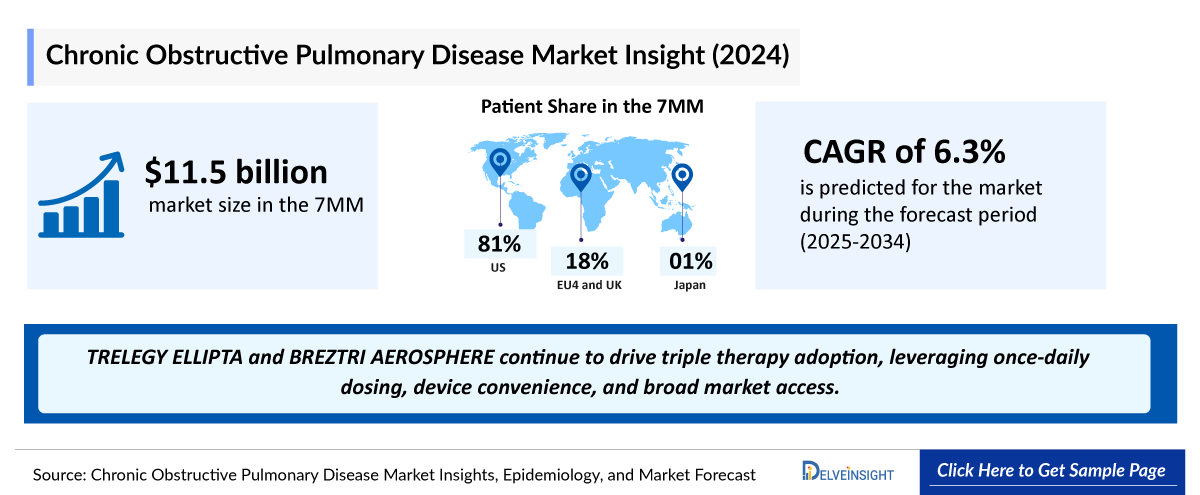
- The Chronic Obstructive Pulmonary Disease market in the 7MM is projected to grow at a CAGR of 6.3% by 2034 in the leading countries (US, EU4, UK, and Japan).
COPD Market and Epidemiology Analysis
- COPD involves structural lung damage from chronic inflammation, often beyond just smoking, with nearly one-fourth of COPD patients linked to other risk factors like environmental exposures and early-life insults/injuries (utero and during childhood). Women, rural populations, and those with low Socioeconomic Status (SES) face higher disease burden, lower diagnosis rates, and worse disease outcomes, highlighting underdiagnosis and the need for personalized, inclusive care.
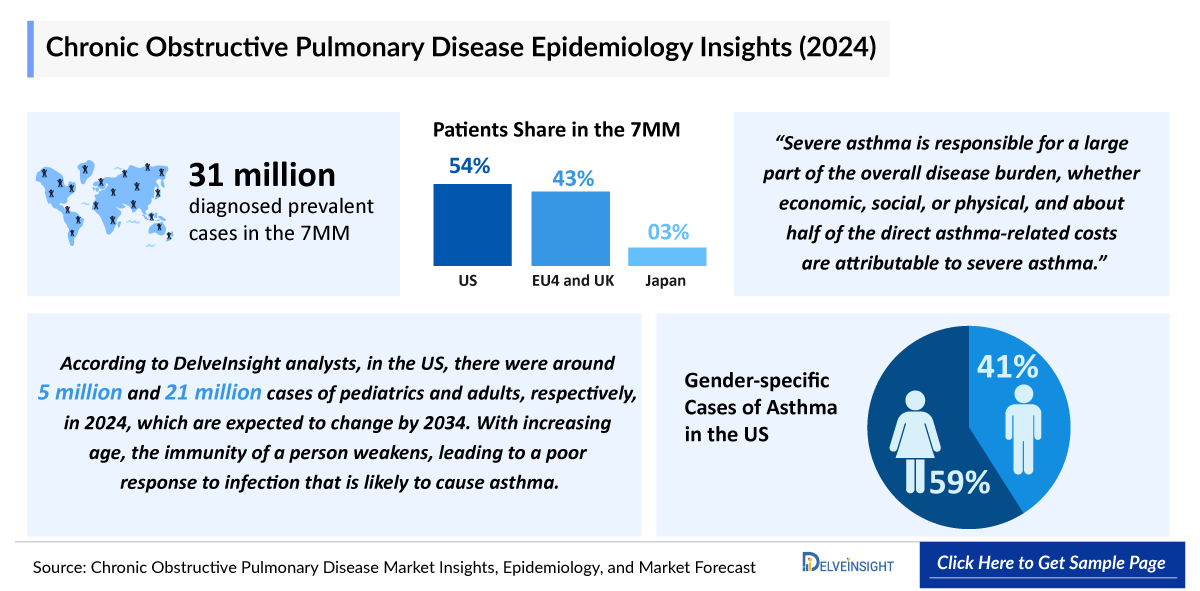
- In 2024, among the 7MM, the US had the highest number of diagnosed prevalent cases of COPD. In the US, there were ~16.5 million diagnosed prevalent cases of COPD in 2024.
- The patient volume growth of COPD is projected to increase over the coming years due to a combination of rising incidence, continued exposure to COPD risk factors, increased diagnosis and awareness rates, and an aging population.
- Historically, COPD treatment has evolved to center around bronchodilator-based regimens, with LAMAs (Long-acting Muscarinic Antagonists) and LABAs (Long-acting Beta-agonists) forming the treatment backbone of COPD. Double therapy (LAMA/LABA) is preferred for moderate symptoms, and triple therapy (LAMA/LABA/ICS) is reserved for severe COPD or those with frequent exacerbations.
- Since 2024, biologics and dual PDE3/4 inhibitors have been introduced for treating COPD patients. The European Medicines Agency (EMA) is the first regulatory authority in the world to approve DUPIXENT (July 2024) for patients with COPD. In the United States, biologics such as DUPIXENT (September 2024) and NUCALA (May 2025) have been approved to treat COPD patients with an eosinophilic phenotype, whereas OHTUVAYRE, a dual PDE3/4 inhibitor, is approved (June 2024) as a maintenance treatment for COPD.
- The US market is set for a major shift under the Inflation Reduction Act (IRA). Five major COPD therapies, such as TRELEGY ELLIPTA, ANORO ELLIPTA, SYMBICORT, BREO ELLIPTA, and SPIRIVA RESPIMAT, have been selected for the second round of Medicare price negotiations, with high price cuts anticipated in 2027.
- In March 2025, DUPIXENT (dupilumab) was approved in Japan as the first biologic treatment for COPD. The approval was based on positive results from the Phase III BOREAS and NOTUS trials.
- OHTUVAYRE becomes the “first new mechanism of action” to get regulatory nod to treat COPD in more than 20 years. With its growing sales, strong uptake, and international market expansion beyond the US.
- The emerging drug development pipeline features candidates such as depemokimab (GSK), itepekimab (Regeneron Pharmaceuticals/Sanofi), tanimilast (Chiesi Farmaceutici S.p.A.), and others.
Key Factors Driving the Chronic Obstructive Pulmonary Disease Market
Rising Prevalence of COPD
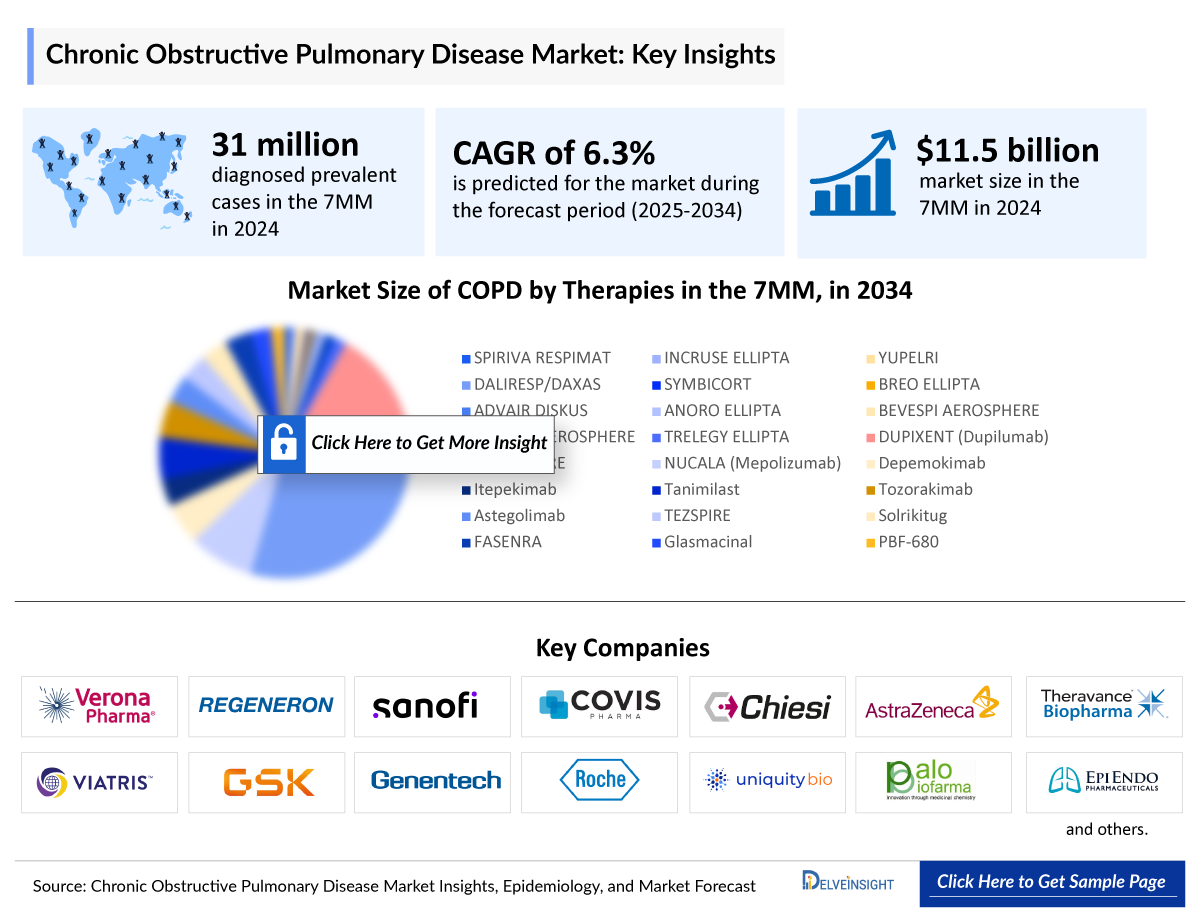
COPD is a leading cause of morbidity and mortality globally. In 2024, the total number of diagnosed prevalent cases of COPD in the 7MM was around 31 million. These figures are expected to reach 36.6 million by 2034. This patient volume growth of COPD is due to a combination of rising incidence, continued exposure to COPD risk factors, increased diagnosis and awareness rates, and an aging population.
Impact of the Inflation Reduction Act on the US COPD Market
The US Chronic Obstructive Pulmonary Disease market is set for a major shift under the Inflation Reduction Act (IRA). Five major COPD therapies, such as TRELEGY ELLIPTA, ANORO ELLIPTA, SYMBICORT, BREO ELLIPTA, and SPIRIVA RESPIMAT, have been selected for the second round of Medicare price negotiations, with high price cuts anticipated in 2027.
Recent Advances in the COPD Market
The recent approval of biologics such as DUPIXENT, NUCALA, and OHTUVAYRE signals a pivotal shift in COPD management, introducing targeted therapies that address underlying inflammation. Alongside a growing pipeline, these advances offer the potential to significantly enhance disease control, improve quality of life, and fill longstanding gaps in treatment for specific patient subgroups.
Strong Late-stage COPD Clinical Trial Activity
The current late-stage drugs for COPD are ones to watch, specifically the potential introduction of biologics to the market. Some of the COPD drugs in late-stage clinical trials include Depemokimab (GSK), Itepekimab (Regeneron Pharmaceuticals/Sanofi), Tanimilast (Chiesi Farmaceutici S.p.A.), Tozorakimab (AstraZeneca), Astegolimab (Roche (Genentech)/Amgen), TEZSPIRE (AstraZeneca/Amgen), FASENRA (AstraZeneca), and others.
DelveInsight's ‘Chronic Obstructive Pulmonary Disease (COPD) – Market Insights, Epidemiology and Market Forecast – 2034’ report delivers an in-depth understanding of the COPD, historical and forecasted epidemiology as well as the COPD market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The COPD market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM COPD market size from 2020 to 2034. The report also covers current COPD treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Chronic Obstructive Pulmonary Disease Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
COPD Epidemiology |
Segmented by
|
|
COPD Companies |
|
|
COPD Therapies |
|
|
COPD Market Segmentation |
Segmented by
|
|
COPD Market Analysis |
|
Chronic Obstructive Pulmonary Disease (COPD): Understanding and Treatment Algorithm
Chronic Obstructive Pulmonary Disease (COPD) Overview
COPD is a heterogeneous lung condition marked by chronic respiratory symptoms such as shortness of breath (dyspnea), coughing, mucus production (expectoration), and/or recurrent flare-ups (exacerbations). It results from structural abnormalities in the airways, including bronchitis and bronchiolitis, and/or the alveoli (as seen in emphysema), leading to persistent and often progressive airflow limitation.
The main risk factor for COPD is tobacco smoking, but other environmental exposures, such as biomass fuel exposure and air pollution, may contribute. Besides exposures, host factors predispose individuals to develop COPD. These include genetic abnormalities, abnormal lung development, and accelerated aging. The airflow limitation is usually measured by spirometry, as this is the most widely available and reproducible test of lung function. Many previous definitions of COPD have emphasized the terms “emphysema” and “chronic bronchitis.” COPD is a leading cause of morbidity and mortality worldwide that induces an economic and social burden that is both substantial and increasing.
Chronic Obstructive Pulmonary Disease (COPD) Diagnosis
The diagnosis of COPD is based on the presence of persistent respiratory symptoms such as chronic cough, sputum production, and breathlessness, particularly in individuals with risk factors like smoking or exposure to environmental pollutants. The gold standard for confirming the diagnosis is spirometry, which demonstrates irreversible airflow limitation, specifically a post-bronchodilator FEV₁/FVC ratio of less than 0.70. Additional assessments may include chest X-rays or CT scans to exclude other lung conditions, and blood tests to check for arterial blood gases or alpha-1 antitrypsin deficiency in younger or non-smoking patients. Tools like the COPD Assessment Test (CAT) and the modified Medical Research Council (mMRC) dyspnea scale help evaluate symptom severity, while the GOLD classification is used to stage the disease and guide management.
Further details are provided in the report.
Chronic Obstructive Pulmonary Disease (COPD) Treatment
COPD management involves relieving symptoms, preventing exacerbations, and slowing disease progression. Key steps include smoking cessation, avoiding pollutants, and staying up to date with vaccinations (influenza, pneumococcal, COVID-19). Bronchodilators are the mainstay of treatment. Short-acting (e.g., salbutamol, ipratropium) provide quick relief, while long-acting agents like formoterol (LABA) or tiotropium (LAMA) are used for maintenance. In patients with frequent exacerbations and high eosinophil counts, Inhaled Corticosteroids (ICS) are added, forming dual or triple therapy (ICS + LABA + LAMA). For patients with uncontrolled Type 2 inflammation, newer biologics like NUCALA (mepolizumab) an IL-5 inhibitor, and DUPIXENT (dupilumab), which blocks IL-4 and IL-13 signaling, can help reduce exacerbations. OHTUVAYRE (ensifentrine) is a novel inhaled therapy that combines bronchodilation and anti-inflammatory action by inhibiting both PDE3 and PDE4 enzymes. Additional options include DALIRESP (roflumilast; PDE4 inhibitor) for chronic bronchitis and mucolytics like N-acetylcysteine. Exacerbations are treated with short-acting bronchodilators, systemic steroids, and antibiotics if infection is suspected. Regular follow-up ensures proper inhaler technique, symptom control, and management of comorbidities.
Further details related to country-based variations are provided in the report.
Chronic Obstructive Pulmonary Disease (COPD) Epidemiology
As the market is derived using a patient-based model, the COPD epidemiology chapter in the report provides both historical and forecasted epidemiology. This includes the total diagnosed prevalent cases of COPD, subtype-specific, gender-specific, age-specific diagnosed prevalent cases, diagnosed prevalent cases of COPD based on the severity of airflow limitation, along with treatment-eligible cases. This analysis spans the 7MM, covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
Key Findings from the Chronic Obstructive Pulmonary Disease Patient Pool Analysis
- The total number of diagnosed prevalent cases of COPD in the 7MM was about ~31 million in 2024.
- In the US, cigarette smoking is the primary cause of COPD, with significantly higher rates observed in both current and former smokers compared to those who never smoked. Additionally, individuals with higher family incomes tend to have lower COPD prevalence, indicating a link between socioeconomic status and disease risk.
- In comparison to the US, in EU4 and the UK, COPD continues to be more prevalent among men, largely reflecting historical patterns of higher smoking rates and occupational exposures in male populations. Although the gender gap has narrowed over time with rising diagnoses in women, men still represent a larger share of the COPD burden.
- In EU4 and the UK, COPD cases were highest in Germany in 2024, whereas the minimum number of cases was in the UK, cases in 2024.
- The US accounted for the highest GOLD 2 cases (~8.2 million), which were followed by GOLD 3 (~4.3 million) cases, compared to other countries in the 7MM.
- In 2024, chronic bronchitis accounted for the highest share of COPD cases in EU4 and the UK, comprising ~6.42 million cases.
- In Japan, there is a significant gap between the estimated number of COPD patients and those receiving treatment, indicating widespread underdiagnosis; this highlights the high unmet need to enhance early detection and management efforts. Japan observed the maximum number of cases from the age group 65–74 years (~283,000) in 2024.
COPD Epidemiology Segmentation
- Total Diagnosed Prevalent Cases of COPD in the 7MM [2020–2034]
- Subtype-specific Diagnosed Prevalent Cases of COPD in the 7MM [2020–2034]
- Gender-specific Diagnosed Prevalent Cases of COPD in the 7MM [2020–2034]
- Age-specific Diagnosed Prevalent Cases of COPD in the 7MM [2020–2034]
- Diagnosed Prevalent Cases of COPD Based on Severity of Airflow Limitation in the 7MM [2020–2034]
- Treatment eligible pool for COPD in the 7MM [2020–2034]
Chronic Obstructive Pulmonary Disease (COPD) Drug Chapters
The drug chapter segment of the COPD Market report encloses a detailed analysis of COPD marketed drugs and late-stage (Phase III, Phase II/III, Phase II, Phase I/II, and Phase I) pipeline drugs. The marketed drugs segment encloses drugs such as NUCALA (GSK Pharmaceuticals), DUPIXENT (Regeneron Pharmaceuticals/Sanofi), and OHTUVAYRE (Verona Pharma), among others. It also helps understand the COPD clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Chronic Obstructive Pulmonary Disease Marketed Drugs
NUCALA: GSK Pharmaceuticals
NUCALA (mepolizumab) is a fixed-dose biologic therapy approved for subcutaneous injection to treat severe eosinophilic asthma, chronic rhinosinusitis with nasal polyps, Eosinophilic Granulomatosis with Polyangiitis (EGPA), Hypereosinophilic Syndrome (HES), and recently, eosinophilic phenotype COPD. NUCALA is available as prefilled syringes, autoinjectors, and powder for injection, designed for easy and flexible use by healthcare providers or patients after training. The medication requires refrigeration and is intended for single-patient use. This delivery system supports convenient, at-home treatment of eosinophilic respiratory diseases.
In May 2025, GSK announced that the US FDA approved NUCALA for use in adults with COPD. The approval was based on data from the METREX and METREO Phase III trials, which showed a statistically significant reduction in moderate and severe exacerbations in patients with elevated eosinophil counts who were already on maximum inhaled therapies.
According to GSK’s 2025 Q1 presentation, the filing for NUCALA in Europe is planned for 2026.
DUPIXENT: Regeneron Pharmaceuticals/Sanofi
DUPIXENT (dupilumab), developed by Regeneron Pharmaceuticals/Sanofi, is primarily developed for the treatment of adult patients with uncontrolled COPD, which is administered SC. DUPIXENT is the first biologic to be approved in Europe, changing the entire pursuit of the market from now on. Recently, DUPIXENT has achieved a significant milestone with its approval as the first-in-world treatment for adults with uncontrolled COPD characterized by elevated blood eosinophils. This decision follows two pivotal Phase III studies, which demonstrated that DUPIXENT effectively reduced exacerbations, enhanced lung function, and improved health-related quality of life.
In September 2024, the US FDA approved DUPIXENT (dupilumab) as an add-on maintenance treatment for adults with inadequately controlled COPD and an eosinophilic phenotype. DUPIXENT is the first biologic medicine approved in the US to treat these patients.
Sanofi and Regeneron have jointly developed DUPIXENT under a global collaboration agreement. Dupilumab has been studied across more than 60 clinical trials involving more than 10,000 patients with various chronic diseases driven in part by Type 2 inflammation.
Apart from its existing approved uses, Sanofi and Regeneron are investigating dupilumab in Phase III trials for various conditions influenced by Type 2 inflammation or allergic reactions.
OHTUVAYRE: Verona Pharma
OHTUVAYRE is a first-in-class selective dual inhibitor of the enzymes PDE3 and PDE4 that combines bronchodilator and nonsteroidal anti-inflammatory effects in one molecule. OHTUVAYRE met the primary endpoint in both ENHANCE-1 and ENHANCE-2, demonstrating statistically significant and clinically meaningful improvements in lung function. A fixed-dose combination of ensifentrine and glycopyrrolate, a LAMA, is currently under development for the maintenance treatment of COPD. Ensifentrine has potential applications for development in noncystic fibrosis bronchiectasis, cystic fibrosis, asthma, and other respiratory diseases.
In July 2025, Merck announced its acquisition of Verona Pharma, adding OHTUVAYRE to its portfolio, with the drug expected to fuel growth into the next decade.
In June 2024, the FDA approved Verona Pharma’s OHTUVAYRE (ensifentrine) for the maintenance treatment of COPD in adult patients.
Comparison of Marketed Drugs Under Development | ||||
|
Drug Name |
Company |
RoA |
MoA |
US First Approval |
|
NUCALA |
GSK |
SC |
IL-5 inhibitor |
2025 |
|
DUPIXENT |
Regeneron Pharmaceuticals /Sanofi |
SC |
IL-4R alpha subunit |
2024 |
|
OHTUVAYRE |
Verona Pharma |
Oral inhalation |
PDE 3 and PDE 4 inhibitors |
2024 |
|
BREZTRI AEROSPHERE/ TRIXEO AEROSPHERE |
AstraZeneca |
Oral inhalation |
ICS+LAMA +LABA |
2020 |
|
DUAKLIR PRESSAIR |
Covis |
Oral inhalation |
LAMA+LABA |
2019 |
|
YUPELRI |
Viatris |
Oral inhalation |
LAMA |
2018 |
|
TRIMBOW |
Chiesi Farmaceutici S.p.A. |
Inhalation |
ICS+LAMA +LABA |
2017 |
|
TRELEGY ELLIPTA |
GSK |
Oral inhalation |
LAMA + LABA + ICS |
2017 |
Chronic Obstructive Pulmonary Disease Emerging Drugs
Depemokimab: GSK
Depemokimab is the first ultra-long-acting biologic to be evaluated in a Phase III trial with a binding affinity and high potency for IL-5, enabling 6-month dosing intervals for patients. IL-5 is known to be a key cytokine (protein) in Type 2 inflammation.
As per GSK’s Q1 presentation, the Phase III clinical trial of depemokimab is expected to start in H2 2025.
Itepekimab: Regeneron Pharmaceuticals/Sanofi
Itepekimab is a fully human mAb that binds to and inhibits IL-33, an initiator and amplifier of broad inflammation in COPD. IL-33 is thought to be involved in different types of inflammation and is particularly elevated in the lungs of former smokers. Sanofi and Regeneron are jointly developing Itepekimab under a global collaboration agreement.
- In May 2025, Sanofi announced the AERIFY-1 Phase III study evaluating itepekimab in former smokers with inadequately controlled COPD met the primary endpoint of a statistically significant reduction in moderate or severe acute exacerbations. The AERIFY-2 Phase III study did not meet the same primary endpoint, although a benefit was seen earlier in the trial.
- In April 2025, Sanofi announced itepekimab’s Phase III readouts in COPD in H2 2025.
- In January 2023, Regeneron announced itepekimab was granted FTD by the US FDA.
TEZSPIRE (tezepelumab): AstraZeneca/Amgen
TEZSPIRE is a first-in-class human mAb that inhibits the action of TSLP, a key epithelial cytokine that sits at the top of multiple inflammatory cascades and is critical in the initiation and persistence of allergic, eosinophilic, and other types of epithelial-driven inflammation associated with severe asthma and other inflammatory diseases.
- In May 2025, AstraZeneca announced presentation of COURSE Phase IIa trial data oF TEZSPIRE at ATS 2025.
- In July 2024, AstraZeneca announced that the US FDA granted a BTD for tezepelumab for the add-on maintenance treatment of patients with moderate-to-very severe COPD characterised by an eosinophilic phenotype.
Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
Depemokimab |
GSK |
III |
Moderate-to-severe COPD with Type 2 inflammation |
SC |
Long-acting IL5 inhibitor |
mAb |
|
Itepekimab |
Regeneron Pharmaceuticals/Sanofi |
III |
Moderate-to-severe COPD |
SC |
IL-33 inhibitor |
mAb |
|
Tanimilast |
Chiesi Farmaceutici S.p.A. |
III |
Moderate or severe COPD and chronic bronchitis |
Inhalation |
PDE4 inhibitor |
Small molecule |
|
Tozorakimab |
AstraZeneca |
III |
Symptomatic COPD with a history of COPD exacerbations, moderate-to-severe COPD, and chronic bronchitis |
SC |
IL-33 inhibitor |
mAb |
|
Astegolimab |
Genentech/ Roche/Amgen |
III |
Moderate-to-severe COPD |
SC |
IL-33 inhibitor |
mAb |
|
TEZSPIRE |
AstraZeneca/Amgen |
III |
Moderate-to-very severe COPD |
SC |
TSLP inhibitor |
mAb |
|
PBF-680 |
Palobiofarma |
II |
Moderate-to-severe COPD |
Oral |
A1AR antagonist |
Small molecule |
|
Solrikitug |
Uniquity Bio |
II |
COPD |
SC |
TSLP inhibitor |
mAb |
Chronic Obstructive Pulmonary Disease Drug Class Insights
IL-5 Inhibitors
IL-5 plays a key role in the growth, activation, and survival of eosinophils, a type of white blood cell involved in certain inflammatory responses. In a subset of COPD patients with eosinophilic inflammation, elevated blood or airway eosinophil levels are linked to increased exacerbation risk and steroid responsiveness. IL-5 inhibitors aim to reduce eosinophilic inflammation, thereby decreasing exacerbations and improving control in these patients.
Currently approved drug NUCALA, binds to IL-5, preventing it from interacting with its receptor on eosinophils while FASENRA an emerging drug, binds to the IL-5 receptor α on eosinophils and attracts natural killer cells to induce eosinophil apoptosis (cell death). IL-5 inhibitors are not yet widely approved or recommended for COPD in current GOLD guidelines, but may be considered in select eosinophilic COPD patients who continue to exacerbate despite triple inhaled therapy.
Research is ongoing to better define biomarkers (like eosinophil thresholds) to predict responders. While IL-5 inhibitors have not revolutionized COPD treatment as they have in severe asthma, they represent a promising biologic option for a niche population of COPD patients with type 2 inflammation.
Dual PDE3/PDE4 Inhibitor
Dual PDE3/PDE4 inhibitors represent a novel class of drugs designed to target both bronchodilation and anti-inflammatory pathways in the treatment of COPD. This dual mechanism aims to address key pathophysiologic features of COPD—airflow limitation (PDE3 inhibition) and chronic inflammation (PDE4 inhibition)─especially in patients with persistent symptoms or frequent exacerbations despite standard inhaled therapy.
OHTUVAYRE (ensifentrine) is a first-in-class inhaled therapy that combines bronchodilation (via PDE3 inhibition) with anti-inflammatory effects (via PDE4 inhibition). It improves FEV₁, symptom scores, and quality of life in COPD patients, making it a valuable maintenance option with a dual mechanism.
Chronic Obstructive Pulmonary Disease (COPD) Market Outlook
The COPD market is expected to grow steadily over the coming years, driven by rising disease prevalence, aging populations, and increased awareness and diagnosis. While traditional inhaled therapies like LABAs, LAMAs, and ICS remain the mainstay, the landscape is evolving with the introduction of novel agents, including biologics and dual-action molecules like PDE inhibitors.
Monotherapy era: ICS, LABA, and LAMA
In the earlier stages of COPD treatment, pharmacological intervention primarily focused on monotherapies, mainly ICS, LABA, and LAMA.
Dual combination therapies: LABA/LAMA + ICS or LABA + LAMA
As understanding of COPD pathophysiology deepened, it became clear that dual combination therapies delivered superior efficacy over monotherapy.
Triple combination therapy: LAMA + LABA + ICS
The progression to triple therapy marked a critical shift in COPD management, particularly for patients not adequately controlled on dual therapy.
Transition toward biologic therapies: Focus on Type 2 inflammation
The COPD treatment paradigm is now undergoing another transformation, with increasing focus on biologic therapies targeting Type 2 inflammation, especially in patients with elevated eosinophil counts.
Treatments such as OHTUVAYRE (ensifentrine) and biologics targeting Type 2 inflammation (e.g, NUCALA, DUPIXENT) are expected to expand therapeutic options, especially for patients with frequent exacerbations or overlapping inflammatory phenotypes. As precision medicine advances and new drugs gain approval, the COPD market is likely to see increased segmentation and a shift toward more personalized, targeted treatment approaches.
- The total market size of COPD in the 7MM was USD 11.5 billion in 2024, due to the rising diagnosed prevalence of COPD, increased awareness of the disease, and a shift in treatment paradigms particularly with emerging biological therapies entering the market. These biologics, being significantly more costly than the current standard of care (SoC), contribute to the overall market expansion in coming years.
- Among all the 7MM, the US captured the highest COPD market with USD ~9.3 billion in 2024.
- NUCALA became the second biologic approved for COPD with eosinophilic inflammation, following closely after DUPIXENT’S breakthrough in 2024.
- TRELEGY ELLIPTA and BREZTRI AEROSPHERE continue to drive triple therapy adoption, leveraging once-daily dosing, device convenience, and broad market access.
- OHTUVAYRE by Verona Pharma is anticipated to capture the maximum market share among emerging therapies by 2034.
- The market for new biologic therapies in COPD is characterized by a smaller eligible patient population, typically focused Type 2 inflammation. However, these biologics have significantly higher prices, often nearly 10 times higher than older branded inhaled therapies, due to their targeted mechanisms, clinical differentiation, and premium positioning.
- The total market size of the COPD treatment market is anticipated to experience growth during the forecast period due to promising emerging treatments that include depemokimab, tanimilast, tozorakimab, TEZSPIRE, solrikitug, FASENRA, and others.
- Approved options such as NUCALA (GSK Pharmaceuticals), DUPIXENT (Regeneron Pharmaceuticals/Sanofi), and OHTUVAYRE (Verona Pharma) play a significant role in managing COPD cases, and the emerging drug development pipeline for COPD includes Depemokimab (GSK), Itepekimab (Regeneron Pharmaceuticals/Sanofi), as well as Tanimilast (Chiesi Farmaceutici S.p.A.), among others.
- The COPD treatment market is growing steadily, driven by the increasing use of biologics, improved diagnosis, and rising awareness. The US accounts for the largest market size of COPD, in comparison to EU4 and the UK (Germany, France, Italy, the UK, and Spain) and Japan.
Further details will be provided in the report….
Chronic Obstructive Pulmonary Disease (COPD) Drugs Uptake
This section focuses on the uptake rate of potential Chronic Obstructive Pulmonary Disease drugs expected to be launched in the market during 2025–2034. The analysis covers the COPD market's uptake by drugs, patient uptake by therapy, and sales of each drug.
This section focuses on the uptake rate of potential Chronic Obstructive Pulmonary Disease drugs expected to be launched in the market during 2025–2034. The landscape of COPD treatment has experienced a profound transformation with the uptake of novel medicines. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, professionals, and the entire healthcare community in their tireless pursuit of advancing care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Further detailed analysis of emerging therapies’ drug uptake in the report…
Chronic Obstructive Pulmonary Disease (COPD) Clinical Trial Analysis
The Chronic Obstructive Pulmonary Disease Market report provides insights into different therapeutic candidates in Phase III, Phase II/III, Phase II, Phase I/II, and Phase I. It also analyzes key Chronic Obstructive Pulmonary Disease companies involved in developing targeted therapeutics.
Chronic Obstructive Pulmonary Disease Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for COPD emerging therapies.
Latest KOL Views on Chronic Obstructive Pulmonary Disease Treatment Landscape
To keep up with current Chronic Obstructive Pulmonary Disease market trends, we take KOLs and SME’s opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights into the evolving COPD treatment landscape. Discussions focused on patient reliance on conventional therapies, the acceptability of therapy switching, and patterns of drug uptake. These experts included individuals with various qualifications and roles such as MD, PhD, instructor, postdoctoral researcher, professor, researcher, and others. They offered valuable insights into the challenges surrounding treatment accessibility.
DelveInsight’s analysts connected with 35+ KOLs to gather insights; however, interviews were conducted with 12+ KOLs in the 7MM. Centers such as the Imperial College, London, UK; University of Michigan, US; Harvard Medical School, USA; University of Ferrara, Italy; University of Marburg, Germany; Kyoto University Respiratory Center, Japan; and University of Milan, Italy, etc. were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or COPD market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying about Chronic Obstructive Pulmonary Disease Patient Trends? | |
|
Region |
KOL Views |
|
United States |
“Dupilumab is the first biologic therapy in COPD to target IL4/IL13-driven Type 2 inflammation, offering hope for patients with persistent exacerbations despite maximal inhaled therapy. In Phase III trials, Dupilumab reduced exacerbations by ~30–34% and improved lung function and quality of life, which marks a significant breakthrough after years without a novel COPD treatment.” |
|
Italy |
“TRILOGY and TRINITY provided definitive real-world and trial evidence that extrafine triple therapy in a single inhaler, TRIMBOW, increases adherence and reduces exacerbations beyond dual or LAMA monotherapy for severe COPD. Our goal is to translate these benefits into routine care, especially for high-risk patients with frequent flare-ups.” |
Chronic Obstructive Pulmonary Disease Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint analysis evaluates multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scores are assigned based on these parameters to assess the overall effectiveness of each therapy.
The analyst analyzes multiple emerging Chronic Obstructive Pulmonary Disease therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chronic Obstructive Pulmonary Disease Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health Technology Assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the 7MM, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The Chronic Obstructive Pulmonary Disease market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies. It also covers programs that improve accessibility and reduce out-of-pocket costs, along with insights into patients insured under federal or state government prescription drug programs, etc.
Chronic Obstructive Pulmonary Disease Market Report Highlights
In the coming years, the COPD market is expected to undergo significant changes due to emerging therapies currently in the pipeline. Additionally, increased healthcare spending across the world is likely to expand the market size, creating greater opportunities for drug manufacturers to increase their market penetration.
The companies and academics are working to assess challenges and seek opportunities that could influence COPD R and D. The therapies under development are focused on novel approaches to treat or improve the disease condition.
The report also encompasses other major segments, i.e., type-specific, gender-specific, age-specific, severity-specific cases of CDI, and the treatment pool of COPD.
The expected launch of potential Chronic Obstructive Pulmonary Disease therapies, Depemokimab, Itepekimab, Tanimilast, Tozorakimab, Astegolimab, TEZSPIRE, FASENRA, and others, might change the landscape in the treatment of COPD.
Scope of the Chronic Obstructive Pulmonary Disease Market Report
- The report covers a segment of key events, an executive summary, a descriptive overview of COPD, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the COPD market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM COPD market.
Chronic Obstructive Pulmonary Disease (COPD) Market Report Insights
- Patient population
- Therapeutic approaches
- COPD pipeline analysis
- COPD market size and trends
- Existing and future market opportunities
Chronic Obstructive Pulmonary Disease (COPD) Market Report: Key Strengths
- Ten-year forecast
- 7MM coverage
- COPD epidemiology segmentation
- Key cross competition
- Highly analyzed market
- Drug uptake
Chronic Obstructive Pulmonary Disease (COPD) Market Report Assessment
- Current treatment practices
- Unmet needs
- Pipeline product profiles
- Market attractiveness
- Qualitative analysis (SWOT and conjoint analysis)
Key Questions Answered in the Chronic Obstructive Pulmonary Disease Market Report
Chronic Obstructive Pulmonary Disease Market Insights
- How does DUPIXENT’s strong efficacy in reducing exacerbations in eosinophilic COPD, combined with its safety profile and biomarker-driven approach, position it ahead of underperforming biologics and therapies like OHTUVAYRE, NUCALA in the evolving COPD treatment landscape?
- Could the upcoming patent expirations of key standard-of-care therapies such as LAMA, LABA, and their combinations trigger accelerated price erosion in the COPD market? As generics begin to enter during the forecast period, how significantly might this affect the market share and profitability of branded drugs?
- Why does triple therapy remain the gold standard for COPD treatment across all severities, and how are BREZTRI and TRELEGY maintaining their leadership in this segment, particularly in reducing all-cause mortality?
- What was the COPD market size, the market size by therapies, market share (%) distribution in 2024, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What are the disease risks, burdens, and unmet needs of COPD? What will be the growth opportunities across the 7MM concerning the patient population with COPD?
- What are the current options for the treatment of COPD? What are the current guidelines for treating COPD in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy Chronic Obstructive Pulmonary Disease Market Report
- The Chronic Obstructive Pulmonary Diseasemarket report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the COPD market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming COPD Companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand KOLs’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Chronic Obstructive Pulmonary Disease companies can strengthen their development and launch strategy.
-market.png&w=256&q=75)

-market%20(2).png&w=256&q=75)



