Common Warts Market
- DelveInsight’s report titled “Common Warts Market Insights, Epidemiology, and Market Forecast – 2034” comprehensively analyzes common warts. The report provides a comprehensive analysis of historical and projected epidemiological data, covering total diagnosed prevalent cases of common warts and gender-specific diagnosed prevalent cases of common warts.
- The Common Warts Prevalence appears to be on the rise, due to increased awareness and diagnosis of these skin lesions, prompting more individuals to seek medical attention. Changes in lifestyle, such as increased exposure to potential risk factors like HPV (Human Papillomavirus) and weakened immune systems, may be fueling the uptick in cases.
- While common warts are widely Common Warts Prevalent, comprehensive epidemiological data remains elusive, with prevalence studies often targeting specific groups and this variability stems from diverse risk factors, sociodemographic elements, and variations in study conditions. Even diagnosis is challenging due to variability in presentation, and lack of defined guidelines.
- The main goal when treating warts is to eradicate the lesions while attempting to minimize pain, avoid scarring, and prevent a recurrence. The current treatment landscape can be divided into three groups, physical or chemical methods, immune-modulating agents, and antiproliferative agents. These include the use of salicylic acid, cryotherapy, use of imiquimod, etc.
- According to DelveInsight analysis, in the current regime, salicylic acid generated more than 50% of the common warts market revenue, in the US in 2023.
- There are no approved products for common warts and none of these therapies are curative, with high chances of disease recurrence. The common wart presents a significant unmet need in terms of effective treatment options. Novel approaches incorporating advanced technologies or immunomodulatory agents hold promise in addressing this gap by targeting the underlying viral infection and bolstering the body's immune response.
- Various therapies are being developed by companies like Nielsen BioSciences, and Verrica Pharmaceuticals, among others that hold promise to change the treatment regime, and significantly impact the common warts market space. These drugs have the potential to be approved during the forecast period, and they are projected to provide a positive impetus to the common warts market dynamics, as they would address a major unmet in the common warts treatment landscape.
Request for Unlocking the Sample Page "Common Warts Treatment Market"
The Common Warts Treatment Market Report provides a comprehensive insight into different facets concerning the patient population, encompassing diagnosis, prescribing trends, physician viewpoints, market accessibility, therapy, and forthcoming market advancements across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan spanning from 2020 to 2034. The Common Warts Treatment Market Report examines current treatment methodologies and algorithms for common warts, assessing the overall market potential, identifying business prospects, and addressing pertinent unmet medical requirements
Common Warts Treatment Market
Common warts, also known as verruca vulgaris, are caused by nonmalignant strains of human papillomavirus (HPV). Common warts are small, grainy skin growths that occur most often on fingers or hands. Rough to the touch, common warts also often feature a pattern of tiny black dots, which are small, clotted blood vessels. It can take a wart as long as two to six months to develop after your skin has been exposed to the virus.
Common warts are easy to distinguish from other types of warts, such as genital, filiform, and plantar warts. They are most commonly found on the hands or fingers, though they can also be found on the knees, ankles, arms, and legs. The majority of warts are asymptomatic. They do, however, produce facial deformity and, in a small percentage of cases, regional discomfort. Most warts do not cause any bothersome symptoms. Some may cause itching, tightness, or a feeling of pressure and might be painful as well, particularly those on the soles of one’s feet. Some warts have small black or brownish dots caused by clotted blood that has leaked from capillaries.
Wart viruses are mainly spread by direct skin contact, but they may also be spread by touching objects like towels or razors. They are more likely to infect moist and soft or injured skin.
Common Warts Diagnosis and Treatment Algorithm
The diagnosis of verruca vulgaris can be made after a small piece of the growth is removed in a procedure called a biopsy or after the entire growth is removed in a procedure called an excision. The tissue is then sent to a pathologist who examines it under the microscope.
Warts usually disappear within a year or two and are little more than an inconvenience. But because they shed virus particles into the surrounding area, they are contagious and cause new warts to appear nearby. In some people, warts may be a more chronic (long-lasting) problem. These people may have individual warts that would not go away, or they keep getting new warts. Warts that continue to persist or grow despite treatment should be examined by a doctor since some skin cancers can masquerade as warts.
Even though most warts go away on their own, some warts require treatment. The goals of treatment are to destroy the wart, stimulate an immune system response to fight the virus, or both. Treatment may take weeks or months. Even with treatment, warts tend to recur or spread.
The current off-label treatment for common warts includes salicylic acid, freezing (cryotherapy), laser treatment, and other antiviral products including drugs such as 5-fluorouracil, and imiquimod which are frequently used in severe and recurrent warts. However, there is a need for effective approved therapy.
Common Warts Epidemiology
The epidemiology section on Common Warts offers an analysis of past and present patient populations, along with projected trends across seven major countries (7MM). Drawing from multiple studies and expert opinions, it aims to elucidate the underlying factors driving current and anticipated trends. Additionally, this segment of the report presents data on the diagnosed patient population, highlighting trends and underlying assumptions.
Various studies have shown that up to 33% of children and teenagers have warts. They are estimated to be much less common in other age groups, affecting only about 3 to 5% of adults. Warts are almost always harmless for people with a healthy immune system.
Key Findings
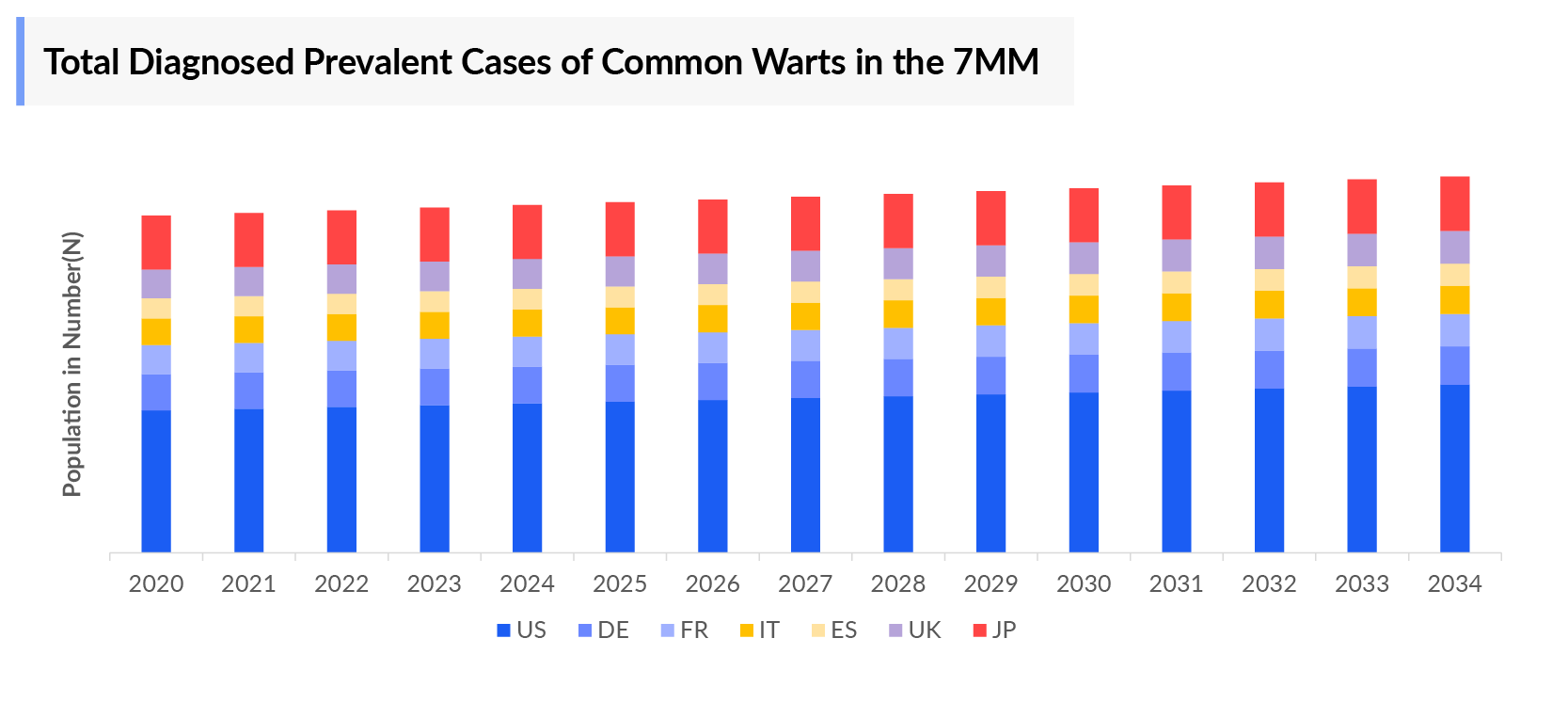
- There were around 14 million Common Warts Diagnosed Prevalent Cases in the 7MM, in 2023. These cases are expected to rise significantly by 2034, due to an increase in disease awareness and improved diagnosis.
- The US had the highest Common Warts Diagnosed Prevalent Cases among the 7MM, accounting for nearly 20% of the total cases in 2023.
- Among EU4 and the UK, Germany accounted for the highest percentage of cases, with nearly 25% of the total cases in EU4 and the UK, followed by France, the UK, Italy, and Spain.
- As per DelveInsight analysis, Japan, accounted for the least Common Warts Diagnosed Prevalent Cases among the 7MM countries, with nearly 1.3 million cases in 2023.
- According to various data available, common warts affect males and females equally, with a very slight difference observed between the two genders.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Common Warts Prevalence
Common Warts Drug Chapters
The drug chapter segment of the Common Warts therapeutics market report encloses a detailed analysis of Common Warts marketed drugs, mid-phase, and late-stage pipeline drugs. It also helps to understand the common warts clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details of each included drug, and the latest news and press releases.
Emerging Therapies for Common Warts
The potential drugs that are expected to launch in the forecasted period include VP-102 by Verrica Pharmaceuticals and CANDIN by Nielsen BioSciences and Maruho.
- CANDIN: Nielsen BioSciences/Maruho
CANDIN is being developed by Nielsen BioSciences, and is currently being assessed in a pivotal Phase III study, patient enrollment for which was initiated recently in March 2024. The goal of the clinical trial is to compare outcomes in healthy subjects 12 years of age and older with at least 3, but no more than 20, common warts (Verruca vulgaris) following treatment with CANDIN or placebo. CANDIN is already approved as a skin test antigen for the assessment of cellular hypersensitivity to Candida albicans. The drug had encouraging phase II results for common and non-common warts among adults.
Neilson has a license agreement with Maruho, granting it exclusive rights to market CANDIN in Japan for the treatment of Verruca vulgaris.
- VP-102: Verrica Pharmaceuticals
VP-102, being developed by Verrica Pharmaceutical, is a drug-device combination product with a topical cantharidine 0.7% solution that is manufactured according to Current Good Manufacturing Practices, delivered via a single-use applicator for precise topical dosing and targeted administration. Verrica has announced positive top-line results from its COVE-1 Phase 2 open-label clinical study of VP-102 for the treatment of common warts.
In January 2024, it received the minutes from the Type C meeting with the US FDA, which was held in November 2023, to discuss the Phase III clinical development plan for YCANTH (VP-102) for the treatment of common warts. Verrica believes that the Type C meeting satisfied its objective of gaining the US FDA’s input on the overall design of a pivotal Phase III study of YCANTH (VP-102) that would support an efficacy supplement for the proposed indication of common warts. The Company will be seeking additional feedback from the US FDA on its updated clinical design in the second quarter of 2024.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
CANDIN |
Stimulate the immune system |
Intradermal |
Nielsen BioSciences/ Maruho |
III |
|
VP-102 |
Degradation of desmosomes in the epidermis |
Inhalation |
Verrica Pharmaceuticals |
III |
|
XXX |
XXX |
Oral |
XXX |
III |
Note: Detailed emerging therapies assessment and list of products to be continued in the report….
Common Warts Market Outlook
Even though most warts go away on their own, some warts require treatment. The goals of treatment are to destroy the wart, stimulate an immune system response to fight the virus, or both. Treatment may take weeks or months. Even with treatment, warts tend to recur or spread. The treatment strategies can be organized into three groups: physical or chemical destruction, immune-modulating agents, and antiproliferation agents. Even with treatment, warts tend to recur or spread.
Tissue destruction can be achieved by physical as well as chemical means. Physical methods utilize cold temperatures or mechanical exposure and include cryotherapy, curettage, and surgical excision, besides lasers which may be used as well. Chemical compounds with destructive properties include keratolytic agents, such as salicylic acid or lactic acid, and caustic agents, such as silver nitrate.
Salicylic acid is the most commonly used medication for warts and is available as an over-the-counter therapy. Wart medications with salicylic acid work by removing layers of a wart a little bit at a time. Salicylic is available with other formulations and is usually combined with cryotherapy and laser. Freezing works by causing a blister to form under and around the wart, then the dead tissue sloughs off within a week or so. This method often requires to be repeated. The side effects include pain, blistering, and discolored skin in the treated area.
Other acids like trichloroacetic acid also require repeat treatments every week. Side effects are burning and stinging. However, destructive methods nonselectively destroy both infected keratinocytes and surrounding cells, thus usually resulting in a larger wound. In addition, destructive methods are associated with relatively high recurrence rates.
Non-destructive methods aim to destroy the virus itself. It uses antimitotic agents like, bleomycin which is a chemotherapeutic agent used to treat localized warts with minimal systemic absorption is used. However, the efficacy of bleomycin has been widely disputed due to few randomized control trials. Bleomycin is an antimitotic chemotherapy agent that can be used intralesionally to treat recalcitrant warts. 5-Fluorouracil (5-FU) is another antimitotic agent that inhibits DNA and RNA synthesis, which decreases the number of replicating virally infected cells in warts.
Immunotherapy drugs are used to elicit humoral and/or cellular immune responses and thereby eliminate the viral infection responsible for warts. Drugs like cimetidine and ranitidine are used in this case. The current treatment landscape is governed by salicylic acid, the most commonly used medication for warts, and is available as over-the-counter and prescriptive strength. There is a need for new, innovative therapeutic approaches for better treatment outcomes. An increase in emerging new drugs for the treatment of warts will lead to better patient outcomes in the coming years.
The Common Warts Market is witnessing the emergence of several new therapeutic options, with key players such as Verrica Pharmaceuticals, Nielsen BioSciences, and Maruho, among others, driving innovation and research in this field. These advancements offer promising avenues for addressing unmet medical needs and improving outcomes for individuals with common warts.
Note: Detailed market outlook to be continued in the report….
Common Warts Market Segmentation
DelveInsight’s ‘Common Warts – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future common warts market, segmented within countries and by therapies. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
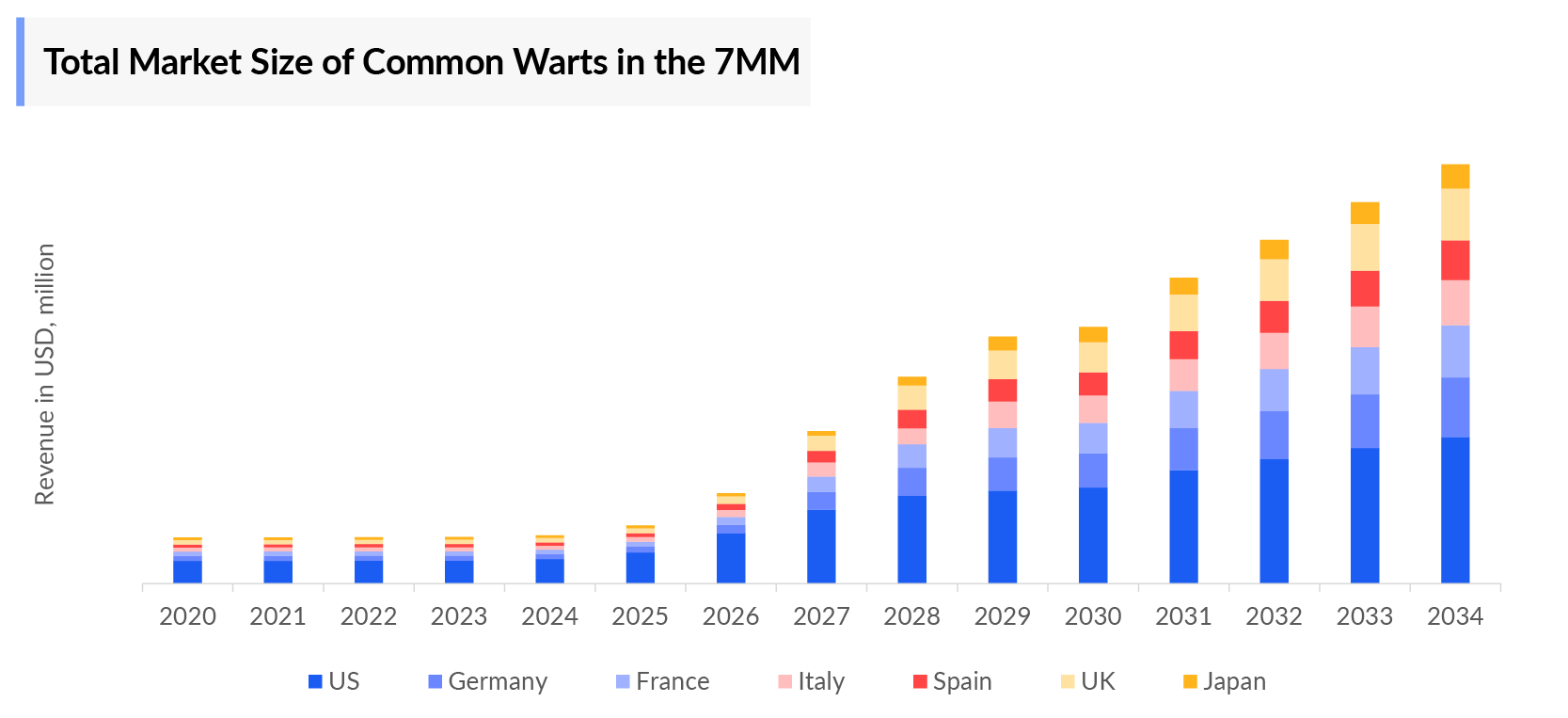
Common Warts Market Size by Countries
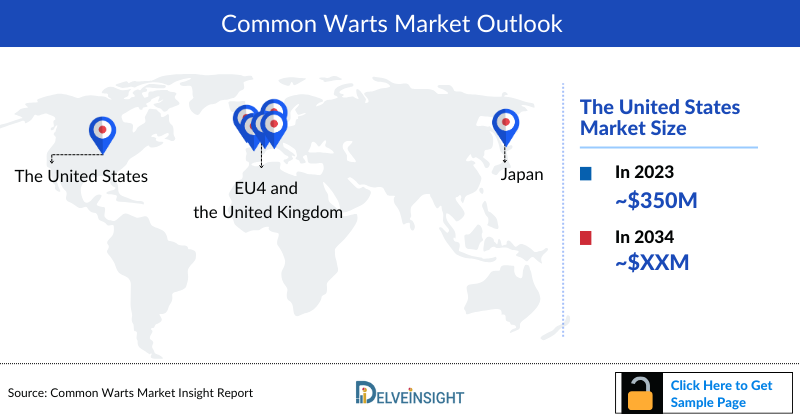
The total common warts market size is analyzed for individual countries (the United States Market, EU4 (Germany, France, Italy, and Spain) and the UK market, and Japan). The United States accounted for a larger portion of the 7MM market for common warts in 2023 due to the high prevalence of the condition and high treatment cost. This dominance is predicted to continue with the potential early entry of new products.
Country-wise Market Size Distribution of Common Warts
Common Warts Market Size by Therapies
The current Common Warts Market is currently dominated by off-label therapies, like salicylic acid, other topical medications like imiquimod, and others like liquid nitrogen or acids like injecting bleomycin or candida antigen. Etc. With many therapies in the pipeline, there is potential that the treatment landscape might see some major changes. It is believed that the common warts treatment market could change by 2034, in the US with the launch of novel therapies with better and broader efficacy, treatment duration, better dosages, and enhanced convenience.
Key Findings
- The Common Warts Treatment Market Size in 7MM was nearly USD 700 million in 2023, which is further expected to increase by 2034 at a significant CAGR for the study period (2020–2034), due to increasing awareness of the disease and launch of the novel therapies in the market.
- The US accounts for the largest Common Warts Treatment Market Size accounting for around 50% of the total 7MM market size, while Japan had the least revenue, due to a smaller patient population.
- The current regime has off-label therapies, of which salicylic acid accounts for a significant proportion. The Common Warts Drugs Market for the current therapies is projected to change with the entry of novel products.
- Various Common Warts Therapies that are likely to be approved include VP-102 by Verrica Pharmaceuticals and CANDIN by Nielsen BioSciences and Maruho, which have the potential to create a significant positive shift in the common warts market size.
- CANDIN is already approved as a skin test antigen for the assessment of cellular hypersensitivity to Candida albicans. The drug had encouraging phase II results for common warts and is currently in Phase III of development.
Note: Detailed market segment assessment will be provided in the final report.
Common Warts Drugs Uptake
This section focuses on the sales uptake of potential common warts drugs that have recently launched or are anticipated to be launched in the common warts market between 2020 and 2034. it estimates the market penetration of the common warts drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the drug’s probability of success (PoS) in the common warts market. For example, for CANDIN, we expect a significant uptake, with a probability-adjusted peak share that is noteworthy, once the drug is launched in the US market in the forecast period.
Note: A detailed assessment of drug uptake will be provided in the full report.
Common Warts Market Access and Reimbursement
DelveInsight’s ‘Common Warts Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of common warts. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current common warts market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the common warts domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or common warts market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the common warts' unmet needs.
Common Warts: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as California Dermatology Institute; University of Michigan, US, University of Catania, Italy; American Academy of Dermatology; Queen Mary University of London, United Kingdom; Osaka University, Japan; University Hospital Birmingham; and others.
“Most individuals are infected with human papillomavirus (HPV) at least once in their lifetime. Infections with low-risk types can cause genital warts, whereas high-risk types can cause malignant tumors”.
Note: Detailed assessment of KOL Views will be provided in the full report
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the common warts Market using various Competitive Intelligence tools, including SWOT analysis, Conjoint analysis, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability. The emerging common warts therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the common warts market.
Note: Detailed assessment of SWOT analysis and conjoint analysis will be provided in the full report
Common Warts Pipeline Development Activities
The Common Warts therapeutics market report provides insights into therapeutic candidates in Phase II and III stages. It also analyzes Common Warts Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Common Warts therapeutics market report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Common Warts therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Common Warts Treatment Drugs
Common Warts Therapeutics Market Report Scope
- The Common Warts therapeutics market report covers a segment of key events, an executive summary, a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines
- Additionally, an all-inclusive account of both the current and emerging therapies along with the elaborative profiles of late-stage and prominent therapies will have an impact on the current Common Warts Treatment Market Landscape
- A detailed review of the Common Warts Treatment Market; historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach
- The Common Warts Treatment Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Common Warts Drugs Market
Common Warts Treatment Market Report Insights
- Patient-based Common Warts Market Forecasting
- Therapeutic Approaches
- Common Warts Pipeline Drugs Analysis
- Common Warts Market Size and Trends
- Existing and future Common Warts Drugs Market Opportunity
Common Warts Report Treatment Market Key Strengths
- 11 Years Common Warts Market Forecast
- 7MM Coverage
- Common Warts Epidemiology Segmentation
- Key Cross Competition
- Attribute Analysis
- Drugs Uptake and Key Common Warts Market Forecast Assumptions
Common Warts Treatment Market Report Assessment
- Current Common Warts Treatment Market Practices
- Common Warts Unmet Needs
- Common Warts Pipeline Drugs Profiles
- Common Warts Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Common Warts Treatment Market Insights
- What was the common warts treatment market size, the Common Warts market size by therapies, and Common Warts drugs market share (%) distribution in 2020, and how it would all look in 2034? What are the contributing factors for this growth?
- What are the unmet needs are associated with the current treatment market of common warts?
- How is PT010 going to contribute to the market of common warts after approval?
- Which drug is going to be the largest contributor in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Common Warts Epidemiology Insights:
- What are the disease risks, burdens, and Common Warts Unmet Needs? What will be the growth opportunities across the 7MM concerning the patient population of common warts?
- What are the historical and forecasted common warts patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Why do only limited patients appear for diagnosis? Why is the current year diagnosis rate not high?
- What factors are affecting the diagnosis and treatment of the indication?
- Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of common warts? What are the current treatment guidelines for the treatment of common warts in the US and Europe?
- How many companies are developing therapies for the treatment of common warts?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of common warts?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Common Warts?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted market of Common Warts?
Reasons to buy
- The Common Warts Treatment Market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Common Warts Market
- Insights on patient burden/disease Common Warts Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- To understand the existing Common Warts Drugs Market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs
- To understand the perspective of key opinion leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Common Warts Drugs Market so that the upcoming players can strengthen their development and launch strategy
Stay Updated with us for Recent Articles








.png&w=3840&q=75)
