Hypoparathyroidism Market
- In 2023, the market size of hypoparathyroidism in the 7MM was estimated to be ~USD 370 million.
- Hypoparathyroidism is a rare disease characterized by low serum calcium levels, elevated serum phosphorus levels, and absent or inappropriately low levels of parathyroid hormone (PTH) in the circulation. The prevalence of hypoparathyroidism in the United States is estimated to be 24 to 37 per 100,000 individuals.
- Hypoparathyroidism most frequently arises due to postoperative complications following thyroidectomy, resulting in inadequate levels of PTH in the body. Nonsurgical etiologies include rare genetic conditions and syndromes, autoimmune destruction of the glands, destruction or invasion due to the tumor, radiation, or infiltration by iron or copper; hypomagnesemia and magnesium depletion; and idiopathic.
- The diagnosis relies on assessing the levels of albumin-corrected total calcium, plasma parathyroid hormone, serum magnesium, and 25-hydroxyvitamin D within the body.
- Low, or inappropriately normal, concentration of serum PTH in association with hypocalcemia is the hallmark of hypoparathyroidism and helps to differentiate this disease from other disorders associated with hypocalcemia (eg, vitamin D deficiency). Hence, a reliable assay (second/third-generation assays) for measuring serum PTH is critical for making the diagnosis.
- The treatment goal is maintaining serum calcium levels in the low-normal range, controlling symptoms, and avoiding hypercalciuria. Standard of care (SoC) consists of dietary and oral calcium supplements, active vitamin D analogs, thiazide diuretics when necessary to help manage hypercalciuria and low salt diet, and magnesium supplementation, in some cases.
- Patients who are more difficult to control on traditional therapy can be treated with daily subcutaneous injections of recombinant human parathyroid hormone. PTH replacement reduces the oral calcium and calcitriol requirements, increases physiological bone turnover, and improves quality of life.
- NATPARA is currently the only approved drug to treat hypoparathyroidism. However, in September 2019, Takeda issued a US recall for all doses of NATPARA for injection due to a potential issue related to rubber particulates and has also planned to discontinue manufacturing the drug globally at the end of 2024.
- Few therapies are being investigated for the treatment of hypoparathyroidism. Some key players involved in the development are Amolyt Pharma (AZP-3601), Ascendis Pharma (TransCon PTH), and Calcilytix Therapeutics (encaleret).
- It has been observed that hypoparathyroidism prevalence varied across the 7MM countries based on the age-specific prevalence of the disease. For instance, the highest proportion was observed in the 65+ years age group in the US, while in EU4 and the UK, people aged 55─64 years accounted for the maximum patient pool. Additionally, it has been observed that hypoparathyroidism manifests in females and males at a ratio of approximately 4:1.
Hypoparathyroidism Market Report Summary
- The report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies and the elaborative profiles of late-stage (Phase III) and prominent therapies that would impact the current treatment landscape and result in an overall market shift has been provided in the report.
- The report also encompasses a comprehensive analysis of the Hypoparathyroidism market, providing an in-depth examination of its historical and projected market size (2020 – 2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The report includes qualitative insights that provide an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM Hypoparathyroidism therapeutics market.
The table given below further depicts the key segments provided in the report:
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Hypoparathyroidism Market |
|
|
Hypoparathyroidisms Market Size | |
|
Hypoparathyroidism Companies |
Ascendis Pharma, Amolyt Pharma, Bridgebio, Calcilytix Therapeutics, and others. |
|
Hypoparathyroidism Epidemiology Segmentation |
|
Hypoparathyroidism Market Insights
Various key players are leading the treatment landscape of Hypoparathyroidism, such as Ascendis Pharma, Amolyt Pharma, Bridgebio/Calcilytix and others. The details of the country-wise and therapy-wise market size have been provided below.
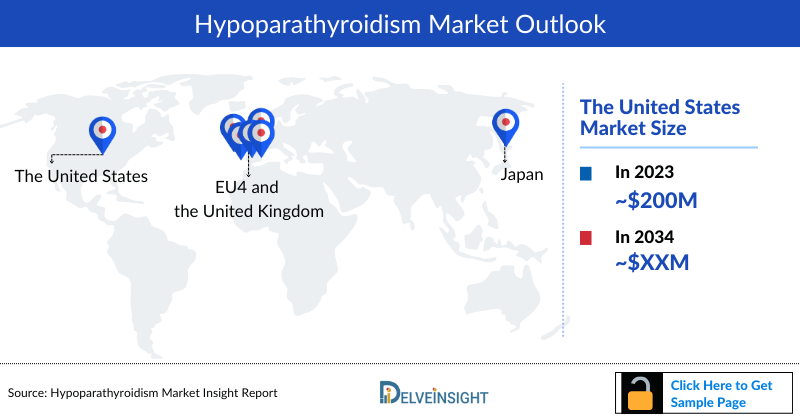
- The United States market size accounted for ~USD 200 million in 2023, constituting approximately 60% of the total 7MM market.
- Among EU4 and the UK, Germany accounted for the largest market size in 2023.
- In 2023, among all the therapies, the highest revenue was generated by Teriparatide, i.e., ~USD 120 million, in the 7MM.
- In the 7MM, TransCon PTH (palopegteriparatide) is expected to achieve the highest revenue by 2034.
Hypoparathyroidism Recent Developments
- In March 2025, the CALYPSO Phase III trial showed that eneboparatide (AZP-3601), an investigational PTH receptor 1 agonist, met its primary endpoint with statistical significance in adults with chronic hypoparathyroidism at 24 weeks, outperforming placebo. The primary endpoint included normalisation of serum calcium levels and independence from active vitamin D and calcium therapy.
- On September 11, 2024, Ascendis Pharma A/S (Nasdaq: ASND) announced that the FDA has granted Orphan Drug exclusivity to YORVIPATH® (palopegteriparatide, developed as TransCon PTH) for seven years. YORVIPATH is designed for the treatment of hypoparathyroidism in adults, a rare endocrine disease caused by insufficient parathyroid hormone, affecting approximately 70,000 to 90,000 people in the United States.
Hypoparathyroidism Drug Chapters
The section dedicated to drugs in the Hypoparathyroidism report provides an in-depth evaluation of late-stage pipeline drugs (Phase III) related to Hypoparathyroidism.
The drug chapters section provides valuable information on various aspects related to clinical trials of Hypoparathyroidism, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Hypoparathyroidism.
Hypoparathyroidism Emerging Therapies
TRANSCON PTH (palopegteriparatide): Ascendis Pharma
TRANSCON PTH is an investigational prodrug of parathyroid hormone (PTH) and is under development as a once-daily hormone replacement therapy. It is designed to restore physiologic levels of PTH. The company believes that this therapy can address both the short-term symptoms and long-term complications of hypoparathyroidism (HP).
The company is evaluating TRANSCON PTH in 76 adults with hypoparathyroidism in the global Phase III PaTHway trial (NCT04701203). In October 2022, the FDA accepted for Priority Review the New Drug Application (NDA) for TRANSCON PTH (palopegteriparatide).
Encaleret (BBP-305/CLTX-305): Bridgebio/Calcilytix Therapeutics
Encaleret is an investigational small molecule antagonist of the calcium-sensing receptor (CaSR) studied in calcium homeostasis disorders, including autosomal dominant hypocalcemia type 1 (ADH1). Individuals with ADH1 have gain-of-function mutations in the CaSR, causing low serum calcium and a range of debilitating symptoms. The drug is a potential first-in-class CaSR antagonist for ADH1, and it is in a Phase III clinical trial for individuals ages 16 and older diagnosed with ADH1.
In June 2021, the US FDA granted Fast Track designation for encaleret for the treatment of autosomal dominant hypocalcemia (ADH1)
Note: Detailed assessment will be provided in the final report of hypoparathyroidism...
|
Drug Name |
Company Name |
MoA |
Molecule Type |
Patient Segment |
Designation |
|
TransCon PTH |
Ascendis Pharma |
Hormone Replacement Therapy |
Recombinant Protein |
Hypoparathyroidism |
Orphan Drug Designation and Priority review |
|
AZP-3601 (eneboparatide) |
Amolyt Pharma |
Parathyroid hormone receptor 1 (PTHR1) agonist |
Small molecule |
Chronic Hypoparathyroidism |
Orphan Drug Designation |
|
Encaleret (BBP-305/CLTX-305) |
Bridgebio/Calcilytix |
Calcium-sensing receptor (CaSR) antagonist |
Small Molecules |
Autosomal Dominant Hypocalcemia (ADH) |
Fast Track Designation and Orphan Drug Designation |
Hypoparathyroidism Market Outlook
The treatment of hypoparathyroidism is directed toward the specific symptoms apparent in each individual and the lab tests. Treatment aims to raise calcium levels to relieve symptoms without causing abnormally high calcium levels in the blood (hypercalcemia) or the urine (hypercalciuria). The specific therapies used may vary depending on the disease severity, the specific symptoms present, an individual’s age and overall health, personal preference, and additional factors.
Oral calcium supplements can increase calcium levels in the blood. However, calcium supplements can cause gastrointestinal side effects at high doses, such as constipation, in some people. Several different types of calcium supplements are available; some brands may work better for certain people. High doses of vitamin D, generally calcitriol, can help the body absorb calcium and eliminate phosphorus. Another form of vitamin D that may be used is ergocalciferol or cholecalciferol; outside the US, doctors use alpha calcidol. If the calcium levels remain low even with treatment, thiazide diuretics can help decrease the amount of calcium lost through the urine. However, some people with hypoparathyroidism, including people who inherited the condition, should not take thiazide diuretics.
Many new molecules with novel mechanisms, like Hormone Replacement Therapy, Parathyroid hormone receptor 1 (PTHR1) agonist, Calcium-sensing receptor (CaSR) antagonist among others, are being developed for the treatment of Hypoparathyroidism by key players like Ascendis Pharma, Amolyt Pharma, and Bridgebio/Calcilytix among others.
In conclusion, despite the lack of appropriate treatment in the current treatment landscape, many potential therapies with novel mechanisms are expected to enter the market, resolving a dire unmet need and leading to significant improvement in the treatment outcome of Hypoparathyroidism patients. Hence, with the upcoming availability of new treatment options and increasing healthcare spending across the 7MM, the treatment scenario is expected to experience significant growth during the forecast period (2024–2034).
Further details are provided in the report...
Hypoparathyroidism Treatment Market
Overview
Hypoparathyroidism is a rare condition in which the parathyroid glands fail to produce sufficient amounts of parathyroid hormone or lack biological activity. The parathyroid glands are part of the endocrine system, the network of glands that secrete hormones into the bloodstream, where they travel to various body areas. These hormones regulate the chemical processes (metabolism) that influence various organs and activities within the body. Hormones are involved in numerous vital processes, including regulating heart rate, body temperature, blood pressure, cell differentiation and growth, and modulation of several metabolic processes.
Further details are provided in the report...
Hypoparathyroidism Diagnosis
The diagnosis of hypoparathyroidism depends, importantly, on the PTH assay. “Two-site” assays for PTH do not detect large, inactive mid- and carboxy-terminal fragments of PTH that constitute most circulating PTH in euparathyroid subjects. The second- and third-generation assays differ from each other in their amino-terminal recognition sites, with the third-generation assay measuring PTH (1-84) rather exclusively.
The second-generation assay for PTH is more widely used and provides excellent discrimination between hypocalcemia due to hypoparathyroidism and the hypocalcemic states of secondary hyperparathyroidism. Surgical Hypoparathyroidism does not usually pose big issues regarding the diagnosis, but it requires an accurate definition of the type (transient vs. permanent), even in established cases.
Further details related to diagnosis are provided in the report...
Hypoparathyroidism Treatment
Careful surgical planning in patients with hyperparathyroidism with preoperative localization of the parathyroid tumor may reduce the likelihood of postoperative parathyroid gland dysfunction. If an extensive surgical procedure is undertaken, preservation with reimplantation of parathyroid tissue should be done. Presurgical optimization of vitamin D is helpful, given that vitamin D deficiency is very common in the older population and patients undergoing parathyroid surgery. Caution with vitamin D replacement prior to surgery is appropriate due to concerns that too much vitamin D could worsen hypercalcemia. Studies have shown that vitamin D supplementation improves bone health in these individuals.
Further details related to treatment and management are provided in the report…
Hypoparathyroidism Epidemiology
The Hypoparathyroidism epidemiology chapter leverages Delveinsight’s epi-based forecasting model of HIT, and provides historical as well as forecasted epidemiology segmented- total Prevalent Cases, total Diagnosed Prevalent, gender-specific, cause-specific, age-specific, type-specific Cases of Hypoparathyroidism in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- US had the highest prevalent population of hypoparathyroidism, accounting for around 45% of the total prevalent cases in the 7MM with ~130,000 cases, followed by the UK, in 2023. On the other hand, Spain had the lowest prevalent population, with ~11,000 cases in 2023
- Japan had ~33,000 prevalent cases of hypoparathyroidism in 2023, which is anticipated to increase at a steady growth rate by 2034.
- Among the major types of hypoparathyroidism, chronic hypoparathyroidism was observed to be more prevalent than transient hypoparathyroidism, accounting for nearly 90% of the total cases.
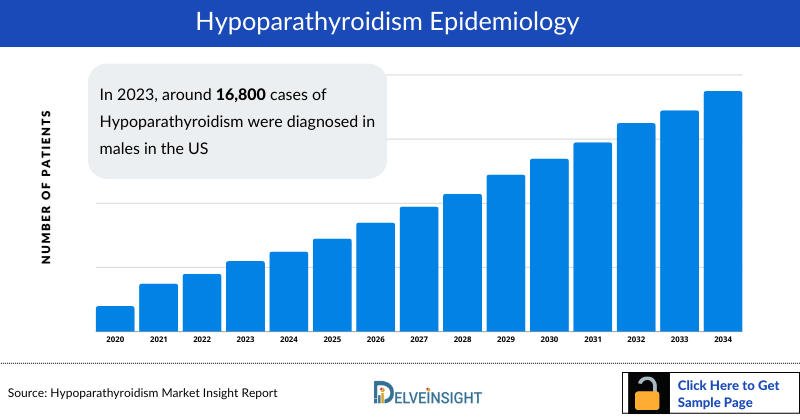
- In 2023, around 16,800 cases of Hypoparathyroidism were diagnosed in males in the US
Further details related to epidemiology will be provided in the report...
KOL Views
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Hypoparathyroidism, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 12 KOLs across the 7MM. We contacted institutions such as the Oregon Health & Science University, University of Florence, Martin-Luther-University, Yale Stem Cell Center, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Hypoparathyroidism therapeutics market, which will assist our clients in analysing the overall epidemiology and market scenario.
Some opinion of experts from various regions has been provided below:
Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyses multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyse the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Hypoparathyroidism, important primary endpoints include albumin-adjusted Serum Calcium (sCa), independence from active vitamin D, among others. Based on these parameters, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, a final weightage score is decided, based on which the emerging therapies are ranked.
Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Hypoparathyroidism Report Insights
- Hypoparathyroidism Patient Population
- Hypoparathyroidism Therapeutic Approaches
- Hypoparathyroidism Market Size and Trends
- Existing Market Opportunity
- Hypoparathyroidism Drugs Uptake
Hypoparathyroidism Report Key Strengths
- Eleven-year Forecast
- The 7MM Coverage
- Hypoparathyroidism Epidemiology Segmentation
- Key Cross Competition
Hypoparathyroidism Report Assessment
- Current Hypoparathyroidism Treatment Practices
- Reimbursements
- Hypoparathyroidism Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
- Hypoparathyroidism Market Drivers
- Hypoparathyroidism Market Barriers
Key Questions Answered In The Hypoparathyroidism Market Forecast Report:
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Hypoparathyroidism management recommendations?
- Would research and development advances pave the way for future tests and therapies for Hypoparathyroidism?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Hypoparathyroidism?
- What kind of uptake will the new therapies witness in coming years in Hypoparathyroidism patients?




.png&w=3840&q=75)
