Lupus Nephritis Market Summary
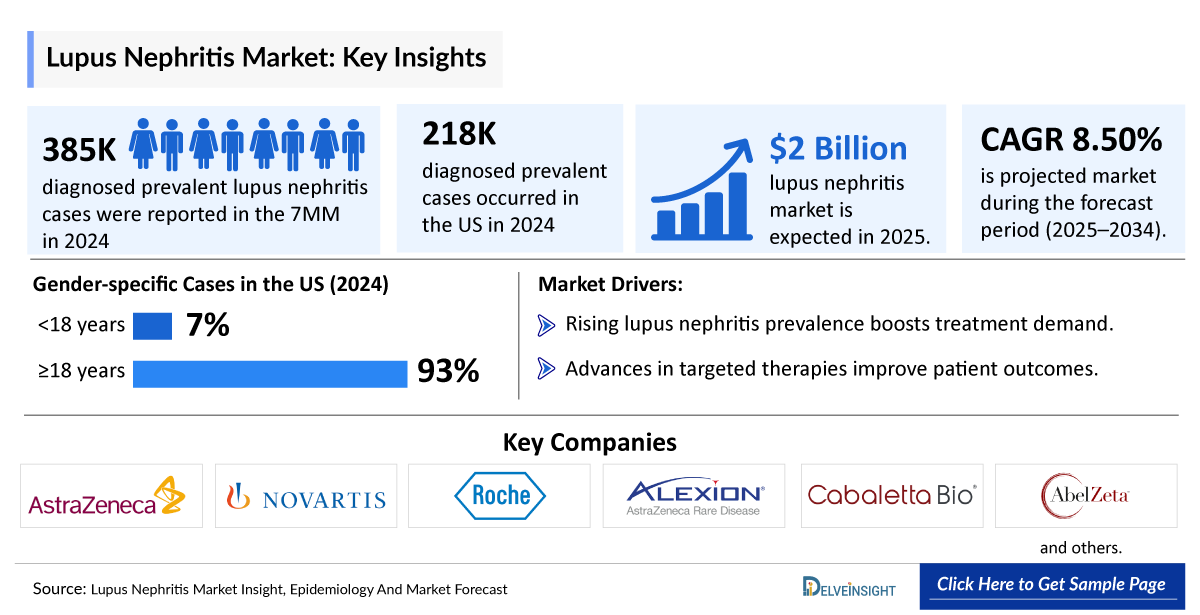
- The Lupus Nephritis Market in the 7MM was valued at approximately USD 1,958 million in 2025 over the forecast period from 2024 to 2034.
- The Lupus Nephritis Market is projected to grow at a CAGR of 8.50% by 2034 in the leading countries (US, EU4, UK, and Japan).
Lupus Nephritis Market and Epidemiology Analysis
- Lupus nephritis is one of the most serious complications of SLE, with the potential to progress to end-stage renal disease (ESRD). It affects approximately 40-60% of SLE patients, and among those, 10-30% may advance to end-stage renal disease (ESRD) despite therapy.
- Lupus nephritis Incidence rate peaked around 2020 and has since declined, while the prevalence rate continues to rise due to improved survival. This suggests a growing but gradually stabilizing patient burden over time.
- Among the histological subtypes, Class IV remains the most prevalent and carries the worst prognosis, often associated with non-remission and high relapse rates. In contrast, Classes I and II are associated with milder disease and better outcomes, whereas Class V, though less common, poses its challenges due to frequent proteinuria and overlap with proliferative forms, reinforcing the need for timely diagnosis and personalized treatment strategies.
- The Lupus Nephritis Diagnosed Prevalent Cases in the 7MM were approximately 385,000 in 2024, and this number is projected to increase by 2034.
- Spain recorded the highest Lupus Nephritis Diagnosed Cases among the EU4 and the UK, suggesting a relatively greater concentration of diagnosed cases within its population.
- Currently, two FDA-approved treatments exist for lupus nephritis: BENLYSTA (belimumab), available as an IV or subcutaneous injection, and LUPKYNIS (voclosporin), the first oral therapy for lupus nephritis.
- Beyond BENLYSTA and LUPKYNIS, a robust and growing pipeline of lupus nephritis therapies is emerging, including agents in late-stage development that may further reshape the standard of care.
- GAZVYA
- Novartis, after securing back-to-back approvals in IgA nephropathy, is now targeting the lupus nephritis space with three therapies expected in the forecast period (2025-2034): ianalumab (BAFF inhibitor), FABHALTA (complement inhibitor), and rapcabtagene autoleucel (CAR-T cell therapy). Ianalumab is likely to debut first, while FABHALTA shows stronger efficacy in early data, and the CAR-T therapy is attracting the most expert buzz for its transformative potential in autoimmune disease.
- The total market size in the 7MM for Lupus nephritis was estimated to be around USD 1,800 million in 2024, dominated by BENLYSTA and LUPKYNIS.
- The total market size of the Lupus nephritis treatment market is anticipated to experience growth during the forecast period due to promising emerging treatments and prevention options that include GAZYVA (obinutuzumab), SAPHNELO (anifrolumab), Ianalumab, and others.
Lupus Nephritis Market Size and Forecast
- 2025 Lupus Nephritis Market Size: USD 1,958 Million
- 2034 Projected Lupus Nephritis Market Size: USD 4,088 Million
- Lupus Nephritis Growth Rate (2025-2034): 8.50% CAGR
- Largest Lupus Nephritis Market: United States
Request for unlocking the Sample Page of the "Lupus Nephritis Market"
Key Factors Impacting the Lupus Nephritis Market Growth
-
Rising Prevalence of Systemic Lupus Erythematosus and Lupus Nephritis
The increasing incidence of SLE, particularly in regions like the United States and Southern Europe, is contributing to a higher number of lupus nephritis cases. In the US, the lupus nephritis prevalent cases are expected to reach ~283K by 2034. This surge underscores the growing demand for effective treatments and diagnostics.
-
Advancements in Biologic and Targeted Lupus Nephritis Therapies
Innovations in biologic treatments, such as voclosporin (LUPKYNIS), belimumab (BENLYSTA), and anifrolumab (SAPHNELO), have revolutionized the management of lupus nephritis. These therapies offer targeted approaches that improve disease control and reduce reliance on traditional immunosuppressants.
-
Increased Lupus Nephritis R&D Investments
Substantial investments in R&D by both public and private sectors are accelerating the discovery of novel therapies. The lupus nephritis clinical trial pipeline is experiencing momentum, with approximately 30 companies, including Roche, Novartis, Alexion Pharmaceuticals, AstraZeneca, Cabaletta Bio, AbelZeta, and others, actively working on developing new treatments. This influx of resources enhances the therapeutic landscape, offering hope for improved patient care.
-
Growing Lupus Nephritis Awareness and Improved Diagnostics
Enhanced awareness among healthcare providers and patients has led to earlier and more accurate diagnoses of lupus nephritis. Advancements in diagnostic techniques, including the use of natural language processing to identify lupus nephritis phenotypes in electronic health records, are facilitating timely interventions and personalized treatment plans.
DelveInsight’s “Lupus Nephritis Treatment Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the Lupus nephritis, historical and forecasted epidemiology as well as the Lupus nephritis market trends in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
Lupus Nephritis Treatment Market Report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Lupus nephritis market size from 2020 to 2034. The report also covers current Lupus nephritis treatment practice/algorithm and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Lupus Nephritis Disease Understanding
Lupus Nephritis Overview
Lupus nephritis is a severe manifestation of Systemic Lupus Erythematosus (SLE), which is an autoimmune disease resulting in chronic inflammation and more than one organ damage, primarily the kidneys. Signs of lupus nephritis include hematuria (blood in the urine) and proteinuria (protein in the urine). Lupus nephritis is more common in women and occurs in patients aged 20–40. Lupus nephritis is categorized into six classes (I–VI), with Classes III (focal proliferative, ~30%), IV (diffuse proliferative, ~40%), and V (membranous, ~20%) being the most prevalent and consistently observed across patient populations. Evaluating kidney function in patients with SLE is crucial, as early identification and treatment of renal impairment can significantly enhance renal prognosis. Lupus nephritis typically occurs within 3 to 5 years of SLE onset and significantly increases the risk of End-stage Renal Disease (ESRD).
Lupus Nephritis Diagnosis
Lupus nephritis is diagnosed using a combination of urine tests, blood tests, and a kidney biopsy. Early indicators may include blood in the urine, foamy urine, elevated blood pressure, and swelling in the feet. Essential diagnostic evaluations include:
- Urine test
- Blood test
- Kidney ultrasound
- Kidney biopsy
Lupus Nephritis Treatment
The treatment of lupus nephritis depends on its class type. Classes 1 and 2 usually require only monitoring. Immunosuppressive and steroid treatment is needed with Classes 3 and 4. In Class 6, where most glomeruli are sclerosed, renal replacement therapy is considered. Active disease in lupus nephritis responds better to treatment than chronic disease. Managing risk factors to prevent progression to CKD or ESRD is essential. Statins are recommended to reduce lipid levels, as CKD raises cardiovascular risk. Antihypertensive therapy with ACE inhibitors or Angiotensin 2 Receptor Blockers (ARBs) is indicated for patients with proteinuria and/or hypertension. Approved therapies for the management of Lupus nephritis include BENLYSTA and LUPKYNIS.
Lupus Nephritis Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Prevalent Cases, Age-specific Diagnosed Prevalent Cases, Diagnosed Prevalent Cases by Class, Total Treated Cases of Lupus Nephritis in the 7MM market covering the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Lupus Nephritis Epidemiological Analysis
- The epidemiology segment also provides the Lupus nephritis epidemiology data and findings across the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- The Lupus Nephritis Diagnosed Prevalent Cases in the US comprised nearly 218,000 cases in 2024 and are projected to increase during the forecast period.
- The Lupus Nephritis Diagnosed Prevalent Cases in the EU4 and the UK were nearly 130,000 in 2024.
- The US contributed the largest Lupus Nephritis Prevalent Population, accounting for roughly ~60% of the 7MM in 2024.
- In EU4 and the UK, the Lupus Nephritis Diagnosed Prevalent Cases were found to be maximum in Spain, followed by the UK. The least number of cases were found in France in 2024.
- In 2024, the Lupus Nephritis Diagnosed Prevalent Cases in the US were distributed as approximately 7% in individuals aged <18 years and around 93% in those aged =18 years.
- In Japan, Class IV was the most prevalent form of lupus nephritis in 2024, with around 18,000 diagnosed cases, reflecting a consistently high disease burden. Class III followed as the second most common subtype, with around 6,800 reported cases.
Lupus Nephritis Drug Analysis
The drug chapter segment of the Lupus Nephritis Therapeutics Market Report encloses the detailed analysis of Lupus nephritis mid and late-stage Lupus Nephritis pipeline drugs. It also helps understand the Lupus nephritis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details of each included drug, and the latest Lupus Nephritis news and press releases.
Lupus Nephritis Marketed Drugs
-
LUPKYNIS (voclosporin): Aurinia Pharmaceuticals/Otsuka Pharmaceuticals
LUPKYNIS is a novel, structurally modified CNI with a dual mechanism of action, acting as an immunosuppressant through inhibition of T-cell activation and cytokine production and promoting podocyte stability in the kidney. The drug has improved near and long-term outcomes in lupus nephritis when combined with a background immunosuppressive therapy regimen for treating adult patients with active lupus nephritis. LUPKYNIS reduces cytokine activation by inhibiting calcineurin and blocks IL-2 expression and T-cell-mediated immune responses. LUPKYNIS was approved by the FDA in January 2021 for the treatment of active lupus nephritis alongside standard immunosuppressive therapy. It is the first oral medication specifically approved by the FDA for lupus nephritis.
-
BENLYSTA (belimumab): GlaxoSmithKline
BENLYSTA, a BLyS-specific inhibitor, is a human monoclonal antibody that binds to soluble BLyS. BENLYSTA does not bind B cells directly. By binding BLyS, BENLYSTA inhibits the survival of B cells, including autoreactive B cells, and reduces the differentiation of B cells into immunoglobulin-producing plasma cells. BENLYSTA was approved for lupus nephritis in the US in 2020 for adults and in 2022 for pediatric patients, followed by approvals in the EU and Japan in 2021.
Lupus Nephritis Emerging Drugs
-
GAZYVA/GAZYVARO (obinutuzumab): Roche
GAZYVA/GAZYVARO (obinutuzumab) injection, for IV use, is an engineered monoclonal antibody that targets a protein called CD20 on the surface of the lymphoma and leukemia cells. It binds to Type 2 CD20 antibodies. This allows obinutuzumab to have a much higher induction of antibody-dependent cytotoxicity and a higher direct cytotoxic effect than the classic CD20 antibodies. Upon binding to CD20, it mediates B-cell lysis through the engagement of immune effector cells by directly activating intracellular death signaling pathways and/or activation of the complement cascade. GAZYVA is marketed as GAZYVARO in Europe. The drug is currently being evaluated in two Phase III trials.
In March 2025, the FDA accepted a supplemental Biologics License Application for Roche’s GAZYVA/GAZYVARO for the treatment of lupus nephritis. Data from the phase III REGENCY study is also being used for the filing submission with the EMA. The FDA’s decision is expected by October 2025.
-
Ianalumab (VAY736): Novartis
Ianalumab is a subcutaneously administered fully human HuCAL antibody that targets BAFF-R and is being investigated by Novartis for the treatment of autoimmune hepatitis, idiopathic pulmonary fibrosis, SLE, and lupus nephritis. It is an anti-BAFF receptor, fully human monoclonal antibody engineered for direct ADCC-mediated B-cell depletion. Currently, the drug is being studied in Phase III of clinical trials.
Novartis anticipates its planned submission scheduled by =2028.
Know more about Anti-CD38 Therapies @ Anti-CD38 Therapies
Lupus Nephritis Drug Class Insights
The management of lupus nephritis is closely guided by its histological classification, as determined by kidney biopsy. While Classes I, II, and VI typically require minimal or supportive treatment, Classes III, IV, and V demand aggressive immunosuppressive therapy to prevent progression to end-stage renal disease, which can affect up to 30% of patients within 15 years if inadequately treated. Traditionally, the backbone of treatment has involved corticosteroids combined with antimetabolites such as mycophenolate mofetil or azathioprine. However, recent advances have shifted the therapeutic landscape toward targeted biologics. Notably, BENLYSTA and LUPKYNIS have emerged as key additions. Both are FDA-approved for lupus nephritis but act through distinct mechanisms. BENLYSTA targets the B-cell activating factor (BAFF), reducing B-cell survival, while LUPKYNIS is a calcineurin inhibitor that modulates T-cell activation and reduces proteinuria. These newer agents represent a significant advancement in Lupus nephritis therapy, offering more precise and potentially better-tolerated options for long-term disease control.
Lupus Nephritis Market Outlook
In the current market, only two FDA-approved therapies are available for Lupus nephritis. Generally condition is managed in two phases: induction to control acute inflammation and maintenance to sustain remission, but real-world outcomes remain suboptimal. Treatment varies widely, but MMF and cyclophosphamide are core induction therapies, while corticosteroids help control flares but have limited long-term benefit alone. There is a shift toward more intensive treatment for Class III/IV and V lupus nephritis, with triple therapy regimens combining glucocorticoids with either MMF or BENLYSTA, MMF and a CNI, or low-dose cyclophosphamide and BENLYSTA. For Class V, the preferred regimen includes glucocorticoids, MMF, and a CNI. As of now, the treatment space is increasingly dominated by therapies like BENLYSTA AND LUPKYNIS, and the pipeline is rapidly evolving. Key players such as Novartis, Roche, AstraZeneca, and others are actively advancing next-generation interventions that have the potential to transform the future of Lupus nephritis treatment and possibly provide curative outcomes.
Lupus Nephritis Market Report Insights
This section includes a glimpse of the Lupus nephritis in the 7MM
- The Lupus Nephritis Market Size in the 7MM is nearly USD ~ 1,958 million in 2025 and is projected to grow during the forecast period (2025–2034).
- According to DelveInsight’s estimates, the largest Lupus Nephritis Market Size is captured by the US in 2024.
- In EU4 and the UK, Spain has the maximum Lupus Nephritis Drugs Market Share, followed by the UK in 2024, while France had the lowest market share.
- The Lupus Nephritis Market Size in Japan was USD ~49 million in 2024, which is expected to rise during the forecast period (2025–2034).
- The upcoming Lupus Nephritis Therapies are expected to combat the current unmet needs faced by patients with Lupus nephritis.
Lupus Nephritis Drugs Uptake
This section focuses on the anticipated uptake of emerging therapies for Lupus nephritis expected to enter the market during the 2020–2034 study period. The analysis covers drug adoption trends, patient uptake across therapeutic approaches, and projected sales performance. One of the most notable developments in this space is the advancement of Calcineurin-inhibitor immunosuppressant- LUPKYNIS, and on the other hand, BENLYSTA, a BLyS-specific inhibitor.
While BENLYSTA, a BLyS-specific inhibitor, marked a milestone as the first biologic approved for children aged 5-17 and adults with lupus nephritis. On the other hand, LUPKYNIS, a next-generation calcineurin inhibitor established itself as the first oral therapy specifically indicated for active Lupus nephritis. LUPKYNIS offers notable advantages over older agents like cyclosporine and tacrolimus, particularly in terms of safety, predictable pharmacokinetics, and long-term renal outcomes.
LUPKYNIS' commercial performance has been strong; net product sales grew by 36% in 2024, reaching USD 216.2 million, up from USD 158.5 million in 2023. Aurinia reports that over 2,300 patients in the US are currently on therapy. Internationally, the drug is gaining momentum through its partnership with Otsuka in Europe and Japan.
Lupus Nephritis Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stages. It also analyzes key Lupus Nephritis Companies involved in developing targeted therapeutics. The report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for Lupus nephritis emerging therapies.
Latest KOL Views on Lupus Nephritis
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps understand and validate current and emerging therapies and treatment patterns, or the Lupus nephritis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
“It is perceived that BENLYSTA is safer than the new calcineurin inhibitor LUPKYNIS and rates BENLYSTA higher on risk- and cost-benefit performance metrics, saying the drug has the greatest overall benefit to lupus nephritis patients when assessing the two drugs head-to-head.”
– Harvard Medical School, Boston, USA
Lupus Nephritis Report Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Lupus Nephritis Market Access and Reimbursement
Lupus nephritis treatments, particularly biologics and immunosuppressants, often require long-term use and come with high costs, posing reimbursement challenges. While traditional therapies like corticosteroids and antimetabolites are generally covered under standard formularies, newer agents such as BENLYSTA and LUPKYNIS face stricter access controls, including step therapy, prior authorization, and high out-of-pocket costs. Payers often favor older, less expensive treatments, despite their toxicity and limited long-term effectiveness. Additionally, the chronic nature of Lupus nephritis means that treatment costs extend over years, making payers cautious about approving newer, premium-priced options. This fragmented reimbursement landscape can limit patient access to innovative therapies and may discourage investment in novel treatments, echoing broader concerns seen in other specialty drug markets.
Scope of the Lupus Nephritis Market Report
- The Lupus Nephritis Therapeutics Market Report covers a descriptive overview of Lupus nephritis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the Lupus nephritis epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for Lupus nephritis is provided, along with an assessment of new therapies that will have an impact on the current treatment landscape.
- A detailed review of the Lupus Nephritis Treatment Market, historical and forecasted, is included in the report, covering the 7MM drug outreach.
- The Lupus Nephritis Therapeutics Market Report provides an edge while developing business strategies by understanding trends shaping and driving the 7MM Lupus Nephritis Drugs Market.
Lupus Nephritis Market Report Highlights
- In the coming years, the Lupus Nephritis Therapeutics Market is set to change due to emerging therapies in the pipeline and incremental healthcare spending across the world, which would expand the size of the market to enable Lupus Nephritis drug manufacturers to penetrate more into the market.
- The Lupus Nephritis Companies and academics are working to assess challenges and seek opportunities that could influence Lupus nephritis R and D. The therapies under development are focused on novel approaches to treat or improve the disease condition.
- The Lupus Nephritis Therapeutics Market Report also encompasses other major segments, i.e., Total Diagnosed Prevalent Cases, Age-specific Diagnosed Prevalent Cases, Diagnosed Prevalent Cases by Class, and Total Treated Cases of Lupus Nephritis.
- The expected launch of potential Lupus Nephritis Therapies such as Anifrolumab, Ianalumab, Obinutuzumab, Iptacopan, and others, might change the landscape in the treatment.
Lupus Nephritis Market Report Insights
- Patient-based Lupus Nephritis Market Forecasting
- Therapeutic approaches
- Lupus nephritis Pipeline analysis
- Lupus nephritis market size and trends
- Lupus Nephritis Drugs Market Opportunities
- Impact of upcoming therapies
Lupus Nephritis Market Report Key Strengths
- 10-year Lupus Nephritis Market Forecast
- 7MM coverage
- Lupus nephritis epidemiology segmentation
- Key cross competition
- Highly analyzed Lupus Nephritis Drugs Market
- Lupus Nephritis Drugs uptake
Lupus Nephritis Market Report Assessment
- Current Lupus Nephritis Treatment Practices
- Lupus Nephritis Unmet Needs
- Lupus Nephritis Pipeline Drugs Profiles
- Lupus Nephritis Drugs Market Attractiveness
- SWOT and conjoint analysis
Key Questions Answered in the Lupus Nephritis Market Report
Lupus Nephritis Market Insights
- What are the patient burden trends of Lupus nephritis in the seven major markets? Historically, why is the prevalence rate of Lupus nephritis increasing while its incidence rate is declining across the 7MM?
- Lupus nephritis is associated with significant morbidity and mortality, particularly among younger women and immunocompromised populations. Each disease flare not only accelerates cumulative kidney damage but also increases the risk of progression to ESRD. What are the renal survival and mortality rates (%) across the 7MM following each lupus nephritis flare or recurrence?
- What is the current standard of care for induction and maintenance therapy in Lupus nephritis across 7MM?
- What was the Lupus Nephritis Drugs Market Share (%) distribution in 2020, and what would it look like in 2034?
- What would be the Lupus Nephritis Treatment Market Size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- What are the key findings about the market across the 7MM, and which country will have the largest Lupus nephritis market size during the study period (2020–2034)?
- At what CAGR, the Lupus nephritis Drugs Market is expected to grow at the 7MM level during the study period (2020–2034)?
- What would be the Lupus nephritis market outlook across the 7MM during the study period (2020–2034)?
- What would be the Lupus nephritis market growth till 2034, and what will be the resultant market size in 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the disease risk, burden, and unmet needs of Lupus nephritis?
- What is the historical Lupus nephritis patient pool in the US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan?
- Why does Spain have the highest prevalence rate of Lupus nephritis among the EU4 and the UK?
- What would be the forecasted patient pool of Lupus nephritis at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Lupus nephritis?
- Out of the above-mentioned countries, which country would have the highest prevalence of Lupus nephritis during the study period (2020–2034)?
- At what CAGR is the population expected to grow across the 7MM during the study period (2020–2034)?
Reasons to Buy Answered in the Lupus Nephritis Report
- The Lupus Nephritis Therapeutics Market Report will help in developing business strategies by understanding trends shaping and driving the Lupus Nephritis Drugs Market.
- To understand the future market competition in the Lupus Nephritis Drugs Market, an insightful review of the key market drivers and barriers.
- Organize sales and marketing efforts by identifying the best opportunities for Lupus nephritis in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Lupus Nephritis Drugs Market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the Lupus Nephritis Drugs Market.
- To understand the future market competition in the Lupus Nephritis Drugs Market.
Stay updated with us for Recent Articles:-