Selective Inhibitor of the CXCR4 Chemokine Receptor Market Summary
- The Selective Inhibitor of the CXCR4 Chemokine Receptor Market Growth is primarily driven by the rising prevalence of cancers, increased adoption of precision medicine, and advancements in drug discovery technologies aimed at selective CXCR4 inhibition.
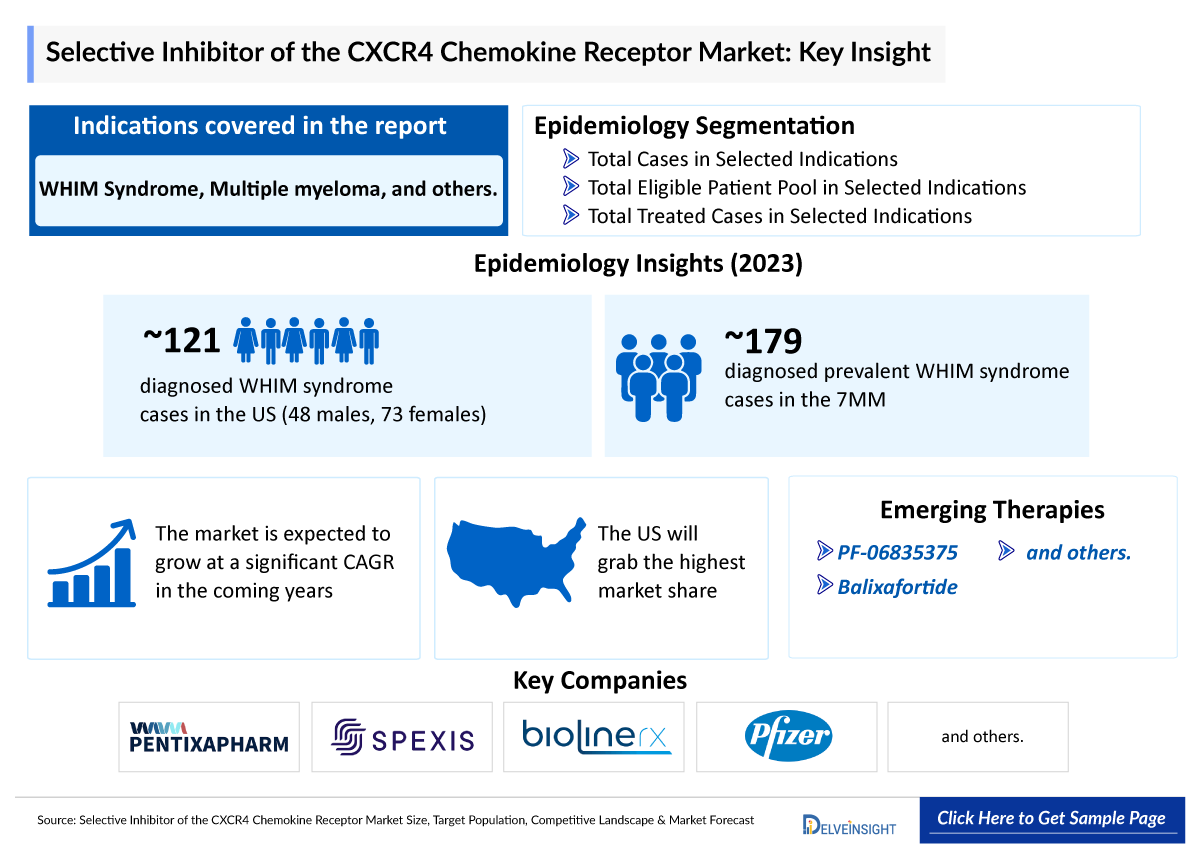
- The leading Selective Inhibitor of the CXCR4 Chemokine Receptor Companies such as X4 Pharmaceuticals, Pfizer, Pentixapharm Holding AG, Spexis, BioLineRx, and others.
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Insights and Forecast
- A selective inhibitor of the CXCR4 chemokine receptor is a compound designed to specifically block the activity of the C-X-C chemokine receptor type 4 (CXCR4). CXCR4 plays a crucial role in various physiological and pathological processes, especially those involving cell trafficking, immune response, cancer metastasis, and stem cell homing. Inhibiting this receptor has therapeutic potential across a range of diseases
- The most recently approved Selective Inhibitor of the CXCR4 Chemokine Receptor by the US Food and Drug Administration (FDA) is mavorixafor, marketed under the brand name XOLREMDI. Developed by X4 Pharmaceuticals, XOLREMDI received the US FDA approval on April 26, 2024, for the WHIM syndrome treatment (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) in patients aged 12 years and older.
- Multiple Selective Inhibitor of the CXCR4 Chemokine Receptor Companies are actively advancing investigational Selective Inhibitor of the CXCR4 Chemokine Receptor across various indications. For example, Pfizer is developing PF-06835375, a biologic designed to be anti-CXCR5. It is being evaluated in a Phase II clinical trial for the Immune Thrombocytopenic Purpura treatment.
- Apart from the Pfizer multiple other companies are developing candidate targeting CXCR4 like Pentixapharm Holding AG, Spexis, and BioLineRx, among others.
- Selective Inhibitor of the CXCR4 Chemokine Receptors hold strong potential as tumor microenvironment modulators that convert immunologically "cold" tumors into "hot" ones by enhancing T-cell infiltration and reducing immune suppression. This positions them as ideal combination partners with checkpoint inhibitors in refractory solid tumors—unlocking new indications, expanding the immuno-oncology market, and enabling biomarker-driven precision therapies.
- There is a significant need for highly selective CXCR4 inhibitors that can effectively target tumors without disrupting normal physiological functions. Given CXCR4's widespread expression in healthy tissues, current inhibitors risk off-target effects, including hematopoietic disturbances and impaired tissue repair. Developing agents that precisely modulate CXCR4 activity in tumor cells while sparing normal cells remains a critical challenge to enhance therapeutic efficacy and safety.
Factors Impacting the Selective Inhibitor of the CXCR4 Chemokine Receptor Market Growth
-
Rising Prevalence of Cancer and HIV/AIDS
The increasing global incidence of cancer and HIV/AIDS is a significant driver for the CXCR4 inhibitor market. CXCR4 plays a crucial role in tumor progression and metastasis, making it a target for therapeutic intervention. For instance, plerixafor has been utilized in stem cell mobilization for hematopoietic stem cell transplantation in cancer patients. Additionally, the growing number of HIV/AIDS cases underscores the need for effective treatments, as CXCR4 antagonists can help in controlling viral replication by blocking the receptor's role in immune cell trafficking.
-
Advancements in Targeted and Precision Medicine
The shift towards personalized medicine is propelling the demand for CXCR4 inhibitors. These inhibitors offer targeted therapeutic options, minimizing off-target effects and enhancing treatment efficacy. In oncology, CXCR4 antagonists are being explored for their potential to disrupt tumor microenvironments and inhibit metastasis. Moreover, the integration of artificial intelligence in drug discovery is accelerating the identification of novel CXCR4-targeted therapies, further fueling market growth.
-
Expansion of Clinical Applications
CXCR4 inhibitors are being investigated for a broadening range of indications beyond their initial uses. In autoimmune diseases, these inhibitors may help modulate immune responses by affecting the migration of immune cells. Additionally, their role in stem cell mobilization is critical for procedures like hematopoietic stem cell transplantation, which is essential for treating various hematological conditions. The ongoing research into these diverse applications is expected to drive the market's expansion
-
Regulatory Support and Orphan Drug Designations
Regulatory agencies are providing support for the development of CXCR4 inhibitors, particularly for rare diseases. For example, Mavorixafor has received orphan drug designation for the treatment of WHIM syndrome, a rare immunodeficiency disorder. Such designations offer benefits like market exclusivity and expedited regulatory pathways, encouraging pharmaceutical companies to invest in the development of CXCR4-targeted therapies
DelveInsight’s “Selective Inhibitor of the CXCR4 Chemokine Receptor Market, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the Selective Inhibitor of the CXCR4 Chemokine Receptor, historical and projected epidemiological data, competitive landscape as well as the Selective Inhibitor of the CXCR4 Chemokine Receptor market trends in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
The Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Selective Inhibitor of the CXCR4 Chemokine Receptor market size from 2020 to 2034. The report also covers current Selective Inhibitor of the CXCR4 Chemokine Receptor treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Selective Inhibitor of the CXCR4 Chemokine Receptor Market |
|
|
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Size | |
|
Selective Inhibitor of the CXCR4 Chemokine Receptor Companies |
|
|
Selective Inhibitor of the CXCR4 Chemokine Receptor Epidemiology Segmentation |
|
Selective Inhibitor of the CXCR4 Chemokine Receptor Disease and Understanding
Selective Inhibitor of the CXCR4 Chemokine Receptor Overview
CXCR4 is a chemokine receptor overexpressed in multiple cancers and immune disorders, playing a key role in cell trafficking, tumor metastasis, immune evasion, and stem cell homing. Selective inhibition of CXCR4 disrupts the CXCL12-CXCR4 axis, leading to mobilization of hematopoietic stem cells, reduced tumor-stroma interaction, and improved immune cell infiltration. Clinically, CXCR4 inhibitors have shown efficacy in stem cell mobilization (e.g., motixafortide) and rare immunodeficiencies like WHIM syndrome (e.g., mavorixafor). Their emerging role in oncology, especially in combination with checkpoint inhibitors, underscores their potential to reshape the tumor microenvironment and overcome resistance in solid tumors. Ongoing trials aim to expand their utility across oncology, fibrosis, and immune diseases, making CXCR4 inhibition a promising precision medicine strategy with broad translational value.
Selective Inhibitor of the CXCR4 Chemokine Receptor Epidemiology
The Selective Inhibitor of the CXCR4 Chemokine Receptor epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total cases in selected indications for Selective Inhibitor of the CXCR4 Chemokine Receptor, total eligible patient pool in selected indications for Selective Inhibitor of the CXCR4 Chemokine Receptor, and total treated cases in selected indications for Selective Inhibitor of the CXCR4 Chemokine Receptor in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034.
WHIM syndrome
- According to DelveInsight’s estimates, in 2020, the US recorded the highest number of diagnosed prevalent cases of WHIM syndrome with approximately 170 cases.
- In 2020, the diagnosed prevalent cases of WHIM syndrome in EU4 and the UK were approximately 40.
Immune Thrombocytopenic Purpura
- As per the secondary research, in the US, the estimated prevalence of ITP was found to be 9.5 per 100,000 people. The prevalence of ITP is higher among adults over the age of 60 years because adults more frequently develop the chronic form of the disease.
Selective Inhibitor of the CXCR4 Chemokine Receptor Epidemiology Segmentation
- Total Cases in Selected Indications for Selective Inhibitor of the CXCR4 Chemokine Receptor
- Total Eligible Patient Pool in Selected Indications for Selective Inhibitor of the CXCR4 Chemokine Receptor
- Total Treated Cases in Selected Indications for Selective Inhibitor of the CXCR4 Chemokine Receptor
Selective Inhibitor of the CXCR4 Chemokine Receptor Drug Analysis
The drug chapter segment of the Selective Inhibitor of the CXCR4 Chemokine Receptor Market Reports encloses a detailed analysis of Selective Inhibitor of the CXCR4 Chemokine Receptors’ marketed drugs and early, mid, and late-stage (Phase I, Phase II and Phase III) pipeline drugs. It also helps understand the Selective Inhibitor of the CXCR4 Chemokine Receptors' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Selective Inhibitor of the CXCR4 Chemokine Receptor news and press releases.
Selective Inhibitor of the CXCR4 Chemokine Receptor Marketed Drugs
-
XOLREMDI (mavorixafor): X4 Pharmaceuticals
XOLREMDI is a CXC chemokine receptor 4 antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
-
- In January 2025, X4 Pharmaceuticals announced that its Marketing Authorization Application (MAA) for mavorixafor for the treatment of WHIM syndrome has been validated for review and is now under evaluation with the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). The EMA previously granted orphan designation to mavorixafor in WHIM syndrome. Decision on MAA expected in 1H 2026.
- In April 2024, the US FDA approved mavorixafor, which is being marketed in the US under the trade name XOLREMDI, for use as an oral, once-daily therapy in patients 12 years of age and older with WHIM syndrome (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes. XOLREMDI is the first drug approved in the US to treat patients with WHIM syndrome. The company launched XOLREMDI in May 2024.
-
APHEXDA (motixafortide): BioLineRx
APHEXDA (motixafortide) is a CXCR4 antagonist with long receptor occupancy (greater than 72 hours) that, in combination with filgrastim (G-CSF), enables mobilization of hematopoietic stem cells to the peripheral blood for collection and subsequent autologous stem cell transplantation in patients with multiple myeloma.
In September 2023, the US FDA has approved APHEXDA (motixafortide) in combination with filgrastim (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma. APHEXDA is administered by injection, for subcutaneous use.
|
Product |
Company |
Indication |
|
XOLREMDI (mavorixafor) |
X4 Pharmaceuticals |
· WHIM Syndrome |
|
APHEXDA (motixafortide) |
BioLineRx |
· Multiple myeloma |
Selective Inhibitor of the CXCR4 Chemokine Receptor Emerging Drugs
-
PF-06835375: Pfizer
PF-06835375 is an investigational monoclonal antibody developed by Pfizer, targeting the chemokine receptor CXCR5. This receptor plays a pivotal role in B-cell trafficking and the formation of germinal centers, processes integral to various autoimmune conditions. As per the company’s pipeline it is in Phase II open-label, multicenter trial for primary immune thrombocytopenia (ITP), and in a Phase I trial in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
-
Balixafortide: Spexis
Balixafortide is a macrocyclic CXCR4 inhibitor which has been studied in clinical trials involving over 500 subjects and in many therapeutic indications such as solid tumors, hematologic malignancy and stem cell mobilization. Although a Phase III trial in metastatic breast cancer read out negatively in mid-2021, Spexis believes there may be considerable therapeutic potential in other indications and is actively assessing same. Spexis is seeking partnerships and investors to support further development efforts of balixafortide and, potentially, back-up compounds which they have identified and characterized arising from their macrocycle portfolio.
List of Emerging Drugs | |||||
|
Drug Name |
Company |
Indication |
ROA |
Phase |
NCT ID |
|
PF-06835375 |
Pfizer |
ITP |
SC |
II |
NCT05070845 |
|
Balixafortide |
Spexis |
- |
- |
- |
- |
|
XX |
XX |
XX |
XX |
XX |
XX |
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Outlook
The Selective Inhibitor of the CXCR4 Chemokine Receptor market is poised for gradual expansion, driven by validated success in stem cell mobilization (e.g., plerixafor) and emerging approvals in rare immune disorders (e.g., WHIM syndrome with mavorixafor). As the axis gains relevance in tumor immune evasion, fibrosis, and viral persistence, the next growth wave hinges on repositioning CXCR4 antagonists as adjuncts in oncology and chronic inflammatory diseases. Key future growth will be shaped by biomarker-driven trials, better selectivity profiles to reduce toxicity, and strategic combinations with checkpoint inhibitors and CAR-T therapies. However, the market remains early-phase heavy, with few late-stage contenders, leaving room for innovation and competitive disruption.
Selective Inhibitor of the CXCR4 Chemokine Receptor Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging Selective Inhibitor of the CXCR4 Chemokine Receptors expected to be launched in the market during 2020–2034.
Selective Inhibitor of the CXCR4 Chemokine Receptor Pipeline Development Activities
The report provides insights into different therapeutic candidates in phase III phase II, and phase I. It also analyzes key players involved in developing targeted therapeutics. The presence of numerous drugs under different stages is expected to generate immense opportunity for Selective Inhibitor of the CXCR4 Chemokine Receptors’ market growth over the forecasted period.
Latest KOL Views on Selective Inhibitor of the CXCR4 Chemokine Receptor Report
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on Selective Inhibitor of the CXCR4 Chemokine Receptors’ evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility. DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM.
Their opinion helps understand and validate current and emerging therapy treatment patterns or Selective Inhibitor of the CXCR4 Chemokine Receptors’ market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
“There’s real excitement around Selective Inhibitor of the CXCR4 Chemokine Receptors, especially for their role in reshaping the tumor microenvironment,” noted one oncology expert. “We’re seeing encouraging signs that these agents can enhance T-cell infiltration and potentially boost response rates when paired with checkpoint inhibitors.” Another immunologist highlighted safety concerns: “The biggest challenge is achieving selectivity—too much CXCR4 blockade, and you risk disrupting normal hematopoiesis.” Still, many experts agree the class is underutilized: “Beyond stem cell mobilization and WHIM syndrome, there's a broader therapeutic window we haven’t fully explored yet, particularly in precision oncology.”
Selective Inhibitor of the CXCR4 Chemokine Receptor Qualitative Analysis Report
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report
- The Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report covers a segment of key events, an executive summary, and a descriptive overview of Selective Inhibitor of the CXCR4 Chemokine Receptor, explaining their mechanism, and therapies (current and emerging).
- Comprehensive insight into the competitive landscape, forecasts, and the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies that will impact the current landscape.
- A detailed review of the Selective Inhibitor of the CXCR4 Chemokine Receptor Drugs Market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM Selective Inhibitor of the CXCR4 Chemokine Receptor market.
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report Insights
- Selective Inhibitor of the CXCR4 Chemokine Receptor Patient Pool
- Selective Inhibitor of the CXCR4 Chemokine Receptor Therapeutic Approaches
- Selective Inhibitor of the CXCR4 Chemokine Receptor Pipeline Analysis
- Selective Inhibitor of the CXCR4 Chemokine Receptor Market Size and Trends
- Existing and future Selective Inhibitor of the CXCR4 Chemokine Receptor Market Opportunity
Selective Inhibitor of the CXCR4 Chemokine Receptor Report Key Strengths
- 10 Years Selective Inhibitor of the CXCR4 Chemokine Receptor Market Forecast
- The 7MM Coverage
- Key Cross Competition
- Selective Inhibitor of the CXCR4 Chemokine Receptor Drugs Uptake
- Key Selective Inhibitor of the CXCR4 Chemokine Receptor Market Forecast Assumptions
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report Assessment
- Current Selective Inhibitor of the CXCR4 Chemokine Receptor Treatment Practices
- Selective Inhibitor of the CXCR4 Chemokine Receptor Unmet Needs
- Selective Inhibitor of the CXCR4 Chemokine Receptor Pipeline Drugs Profiles
- Selective Inhibitor of the CXCR4 Chemokine Receptor Drugs Market Attractiveness
- Selective Inhibitor of the CXCR4 Chemokine Receptor Qualitative Analysis (SWOT)
Key Questions Answered in the Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report
Selective Inhibitor of the CXCR4 Chemokine Receptor Market Insights
- What was the Selective Inhibitor of the CXCR4 Chemokine Receptor Market Size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative Selective Inhibitor of the CXCR4 Chemokine Receptor Market?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for Selective Inhibitor of the CXCR4 Chemokine Receptor evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with Selective Inhibitor of the CXCR4 Chemokine Receptor? What will be the growth opportunities across the 7MM for the patient population of Selective Inhibitor of the CXCR4 Chemokine Receptor?
- What are the key factors hampering the growth of the Selective Inhibitor of the CXCR4 Chemokine Receptor market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for Selective Inhibitor of the CXCR4 Chemokine Receptor?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy the Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report
- The Selective Inhibitor of the CXCR4 Chemokine Receptor Market Report will help develop business strategies by understanding the latest trends and changing dynamics driving the Selective Inhibitor of the CXCR4 Chemokine Receptor market.
- Understand the existing Selective Inhibitor of the CXCR4 Chemokine Receptor Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the attribute analysis section to provide visibility around leading indications.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Selective Inhibitor of the CXCR4 Chemokine Receptor Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles @ New DelveInsight Blogs