Small Cell Lung Cancer Market
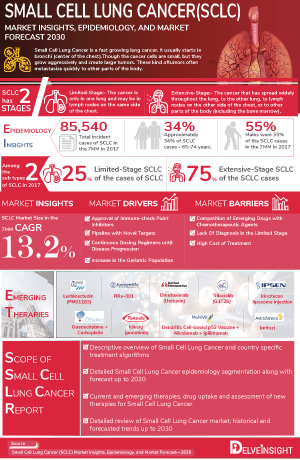
- As per DelveInsight, the Small Cell Lung Cancer Market is expected to expand at a healthy growth rate during the forecast period (2024-2034), owing to the launch of new therapies in the market and the rise in the number of cases.
- Key Small Cell Lung Cancer companies such as Ipsen Biopharmaceuticals (Onivyde), Bristol-Myers Squibb (BMS-986012), Xcovery Holding Company (Vorolanib), EpicentRx (RRx-001), Amgen (AMG 757), and others are evaluating their lead candidates in different stages of clinical development, respectively.
- Lung cancer affects more than 200,000 people in the United States each year and an estimated 2.3 million people worldwide each year. About 10% to 15% of people with lung cancer have a type called Small Cell Lung Cancer.
- The 5-year relative survival rate for people with Small Cell Lung Cancer in the United States is 8% for women and 6% for men.
- Certain treatments available for Small Cell Lung Cancer encompass the usage of IMFINZI (durvalumab) to address extensive-stage- Small Cell Lung Cancer, and ZEPZELCA (lurbinectedin) for the treatment of patients with metastatic Small Cell Lung Cancer.
- About 25% of individuals with limited-stage Small Cell Lung Cancer can be cured with prompt treatment with chemotherapy and radiation therapy.
- The preferred treatment approach, which has been in use for several decades, involves the administration of platinum etoposide. In the conventional approach, topotecan is used as a second-line treatment, particularly for patients who respond well to platinum-based therapy. While topotecan exhibits a response rate of approximately 20%, its impact on improving survival is comparatively modest when compared to the best supportive care available.
- The number and clinical effectiveness of treatment options for Small Cell Lung Cancer lag far behind that of non–Small Cell Lung Cancer, which has seen an explosion of targetable pathways and better responses to immunotherapy.
- In recent times, immunotherapy has emerged as the most promising treatment modality for individuals diagnosed with Small Cell Lung Cancer. Nonetheless, several challenges persist in utilizing these agents effectively, including concerns related to both their toxicity and efficacy. To adequately address the diverse subtypes of this disease, there is a pressing need for additional treatment options.
Download the Sample PDF to Get More Insight @ Small Cell Lung Cancer Market
DelveInsight's “Small Cell Lung Cancer Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of the Small Cell Lung Cancer, historical and forecasted epidemiology as well as the Small Cell Lung Cancer market trends in the United States, EU4 (Germany, France, Italy, Spain) and the United Kingdom, and Japan. Small Cell Lung Cancer market report provides current treatment practices, emerging drugs, market share of individual therapies, and historical and forecasted Small Cell Lung Cancer market size from 2020 to 2034. The report also covers current Small Cell Lung Cancer treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020-2034
Small Cell Lung Cancer Treatment Market
Small Cell Lung Cancer Overview
Small Cell Lung Cancer is an aggressive form of lung cancer. It is characterized by rapid, uncontrolled growth of certain cells in the lungs. Eventually, a tumor forms and the cancer can spread (metastasize) to other areas of the body. The primary risk factor is tobacco use; almost all affected individuals smoke or have a history of smoking. Symptoms can vary from one person to another, and there are rarely any symptoms early in the course of the disease.
Small Cell Lung Cancer Diagnosis
If lung cancer is suspected, the physician will recommend imaging tests (CT, PET, or MRI scans) to identify abnormalities in and around the lungs. Physicians may also take a sample of the mucus to look for cancer cells. If these initial tests identify cancer, a biopsy can be performed by either inserting a needle or making an incision in the chest to remove a small bit of tissue from your lung for further inspection. Another technique physicians commonly use to both visualize and remove lung tissue is called bronchoscopy.
The physician will also determine the extent to which the Small Cell Lung Cancer has spread throughout your body. This descriptive process, called staging, can help inform treatment. Although numerical stages are used for Small Cell Lung Cancer as well as for other cancers, Small Cell Lung Cancer is often classified as either limited-stage disease (LD), where the cancer is confined to a reasonable radiation field within the chest, or extensive-stage disease (ED), where cancer has spread outside the chest.
Small Cell Lung Cancer is rarely detected early. However, with appropriate CT screening for certain patients with a history of smoking, it is occasionally diagnosed before it causes symptoms. Early diagnosis offers the best prognosis for Small Cell Lung Cancer.
Small Cell Lung Cancer Treatment
Small Cell Lung Cancer Treatment options and recommendations depend on several factors, including the type and stage of cancer, possible side effects, and the patient’s preferences and overall health. The most common treatments for Small Cell Lung Cancer are:
- surgery
- radiotherapy
- chemotherapy
- chemotherapy with radiotherapy (chemoradiotherapy)
- immunotherapy
- targeted cancer drugs
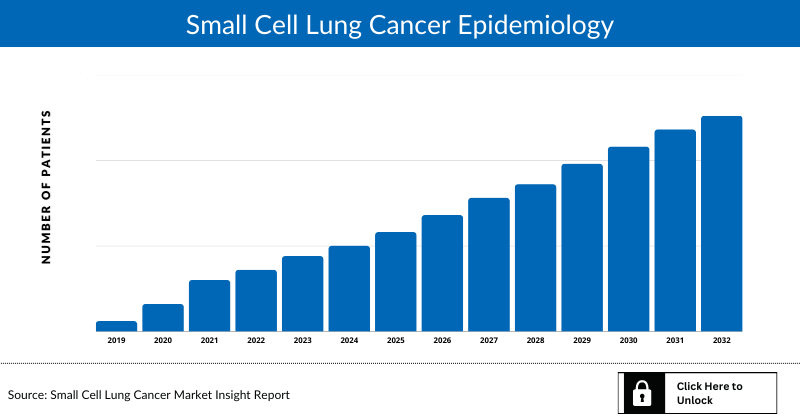
Small Cell Lung Cancer Epidemiology
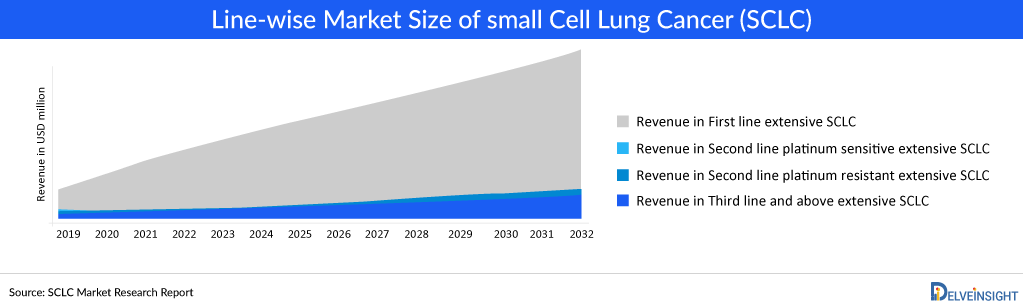
The Small Cell Lung Cancer epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented total incident cases of Small Cell Lung Cancer, gender-specific incident cases of Small Cell Lung Cancer, age-specific incident cases of Small Cell Lung Cancer, line-wise treated cases of Small Cell Lung Cancer in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034. The total incident cases of Small Cell Lung Cancer in the 7MM comprised approximately 93,000 cases in 2022 and are projected to increase during the forecasted period.
- It is estimated that for lung cancer in the US in 2023 are about 238,340 new cases of lung cancer (117,550 in men and 120,790 in women). The society also reported that most lung cancer statistics include both small cell lung cancer and non-small cell lung cancer. About 10% to 15% of all lung cancers are Small Cell Lung Cancer and about 80% to 85% are Non–Small Cell Lung Cancer.
- In the United States, Small Cell Lung Cancer is slightly less common in men (13%) compared to women (14%). The risk of lung cancer increases with age. Both men and women are most likely to be diagnosed with Small Cell Lung Cancer between the ages of 75 and 79.
- In the UK around 15 to 20 out of every 100 lung cancers (around 15 to 20%) diagnosed are this type. It is usually caused by smoking. These cancers tend to spread quite early on.
- It has been observed that about three-quarters of Small Cell Lung Cancer patients are diagnosed with extensive-stage Small Cell Lung Cancer.
- Among the EU4 and the UK, Germany had the highest incident population of Small Cell Lung Cancer, followed by the United Kingdom and France. On the other hand, Spain had the lowest incidence of cases of Small Cell Lung Cancer in 2022.
Get a more detailed overview of How Small Cell Lung Cancer Epidemiology will evolve in the upcoming years: Small Cell Lung Cancer Epidemiology Forecast
- In April 2025, SciTech Development, Inc. announced FDA approval of its IND application to start a Phase 1a/b trial of ST-001, a fenretinide nanoparticle drug, for relapsed/refractory small cell lung cancer (SCLC). This clears the way for patient recruitment and safety evaluation.
- In March 2025, AstraZeneca’s Imfinzi (durvalumab) was approved in the European Union as a monotherapy for adults with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed after platinum-based chemoradiation therapy (CRT).
- In February 2025, Ariceum Therapeutics, a private biotech company based in Berlin, announced that the FDA has granted Orphan Drug Designation (ODD) to its radiopharmaceutical, 225Ac-satoreotide, for treating Small Cell Lung Cancer (SCLC).
- In January 2025, Zai Lab Limited announced that the FDA granted Orphan Drug Designation (ODD) to ZL-1310, a potential first-in-class DLL3 antibody-drug conjugate (ADC), for the treatment of small cell lung cancer (SCLC).
- In January 2025, the FDA cleared the investigational new drug application for SNB-101 as a potential treatment for patients with small cell lung cancer (SCLC), enabling the start of a phase 1b/2 trial. SNB-101 is a novel anticancer drug, marking the first nanoparticle formulation of SN-38, the active metabolite of irinotecan.
- In January 2025, SN BioScience announced that its lead asset, SNB-101, has received Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration (FDA) for a Phase 1b/2 clinical trial. This follows the 2023 orphan drug designation for small cell lung cancer and the Fast Track designation in 2024.
Small Cell Lung Cancer Drug Chapters
The drug chapter segment of the Small Cell Lung Cancer market report encloses a detailed analysis of Small Cell Lung Cancer marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the Small Cell Lung Cancer clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and recent deals and collaborations.
Marketed Small Cell Lung Cancer DrugsZEPZELCA (lurbinectedin): PharmaMar and Jazz Pharmaceuticals
In June 2020, the US Food and Drug Administration granted accelerated approval to ZEPZELCA (lurbinectedin) for adult patients with metastatic Small Cell Lung Cancer with disease progression on or after platinum-based chemotherapy. ZEPZELCA, also known as PM1183, is an alkylating drug that binds guanine residues within DNA. This triggers a cascade of events that can affect the activity of DNA binding proteins, including some transcription factors, and DNA repair pathways, resulting in disruption of the cell cycle and eventual cell death. ZEPZELCA is the first new drug approved for second-line treatment since 1996. ZEPZELCA offers several benefits over the standard therapy, HYMCAMTIN, with one notable advantage being a shorter treatment window. When administering ZEPZELCA intravenously, the process lasts for a mere hour, occurring once every three weeks. Conversely, the older therapy necessitates a more time-consuming regimen, requiring a five-day intravenous course of treatment every three weeks.
IMFINZI (durvalumab): AstraZeneca
In March 2020, the US Food and Drug Administration approved IMFINZI (durvalumab), in combination with etoposide and either carboplatin or cisplatin as the first-line treatment of patients with extensive-stage Small Cell Lung Cancer. IMFINZI is a human monoclonal antibody that binds to PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80, countering the tumor’s immune-evading tactics and releasing the inhibition of immune responses.
Note: Detailed current therapies assessment will be provided in the full report on Small Cell Lung Cancer
Emerging Small Cell Lung Cancer DrugsRRx-001: EpicentRx
RRx-001, which is being investigated by EpicentRx, is a highly selective NLRP3 inhibitor under investigation in a Phase III trial for the treatment of Small Cell Lung Cancer. Currently, the 3L+ Small Cell Lung Cancer REPLATINUM Phase III study is enrolling patients in the US and China. RRx-001, a tumor-activated small molecule that inhibits the NLRP3 inflammasome and repolarizes tumor-associated macrophages (TAMs), is used to restore sensitivity to chemotherapy that has already been tried.
Explore more about the emerging therapies and key companies actively working in the market: Small Cell Lung Cancer Pipeline Insights
Small Cell Lung Cancer Drug Class Insights
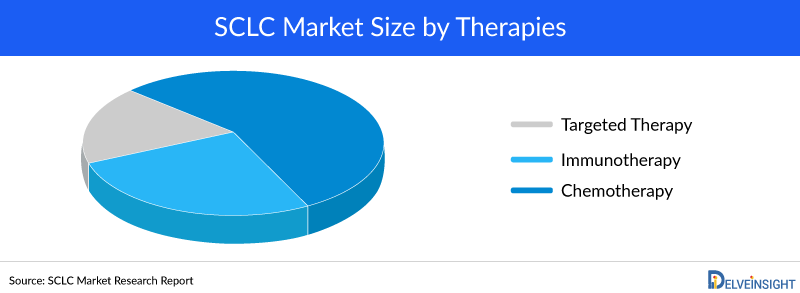
Immunotherapies, including immune checkpoint inhibitors (ICIs), have revolutionized the treatment of various cancers and have emerged as a topic of immense interest in the case of Small Cell Lung Cancer (Small Cell Lung Cancer). Notably, recent advancements have shown improved survival rates in extensive-stage Small Cell Lung Cancer through the incorporation of anti-programmed death ligand 1 (PD-L1) inhibitors alongside platinum-based chemotherapy as the primary treatment. Consequently, the combination of chemoimmunotherapy has now gained official approval as the standard of care.
Researchers are actively exploring the potential applications of ICIs in alternative scenarios as well. For instance, investigations are underway to evaluate the effectiveness of ICIs as consolidation therapy in limited-stage Small Cell Lung Cancer following chemoradiation, as well as in combination with chemoradiation. It is worth noting that the benefits of ICIs in Small Cell Lung Cancer cannot be reliably determined based solely on PD-L1 expression and tumor mutational burden. Consequently, there is an ongoing pursuit for predictive biomarkers that can accurately determine the response to ICIs in Small Cell Lung Cancer.
Innovative immunotherapeutic approaches are currently being investigated in the context of Small Cell Lung Cancer. These approaches are founded upon a comprehensive understanding of Small Cell Lung Cancer biology and the immune tumor microenvironment. Some promising strategies include combinations with inhibitors targeting TIGIT or LAG3, targeting alternative signaling pathways like DNA damage repair, and co-targeting Small Cell Lung Cancer-specific tumor antigens such as fucosyl-GM1 and DLL3. Each subtype of Small Cell Lung Cancer demonstrates unique vulnerability to different therapies, including PARP inhibitors, Aurora kinases, BCL-2, and more.
Few of the emerging therapies have been listed in the below mentioned table:
Small Cell Lung Cancer Market Outlook
For the last three decades, there has been minimal progress in enhancing the survival rates of patients afflicted with Small Cell Lung Cancer. Unfortunately, the clinical outcomes for these patients continue to be unfavorable primarily due to the disease's rapid cell division, propensity for early and extensive metastasis, and the development of resistance to chemotherapy.
There are a number of treatment options for Small Cell Lung Cancer. These treatment options are used to treat a specific patient’s lung cancer that depends on the stage of cancer, the patient’s overall health, including how well the organs of the patient's body are functioning, and the patient's preferences. Surgery, chemotherapy, radiation therapy, and immunotherapy are approved and mainly used treatment choices. The choice of treatment option is dependent on the stage of Small Cell Lung Cancer, symptoms, age, how fast it is growing, and whether patients may suffer various patterns of recurrence requiring subsequent lines of rescue therapies. The most commonly used chemotherapy regimen is Etoposide or Irinotecan plus a platinum-based drug such as Cisplatin or Carboplatin. For people with limited-stage Small Cell Lung Cancer, chemotherapy plus radiation therapy to the chest is given daily over several weeks. People with extensive-stage cancer initially receive chemotherapy for 3 to 4 months or they may receive chemotherapy in combination with immunotherapy.
Initially, the approval of anti-PD-1 agents such as nivolumab (OPDIVO) and pembrolizumab (KEYTRUDA) took place, gradually followed by the approval of atezolizumab (TECENTRIQ) in 2019, and subsequently, durvalumab in 2020, all in the first-line treatment setting. Furthermore, lubenetidin received approval for use in the second-line setting, while trilaciclib was approved for use in the first-line setting to mitigate chemotherapy-induced myelosuppression. However, in 2021, the approval of nivolumab and pembrolizumab in the subsequent-line setting was withdrawn. Nevertheless, they are still utilized in combination with chemotherapy and immune therapies such as atezolizumab and durvalumab in the first-line setting.
Currently, investigators are evaluating novel agents that have the potential to reshape the treatment landscape for extensive-stage Small Cell Lung Cancer, similar to the advancements witnessed in non-Small Cell Lung Cancer. Immune checkpoint inhibitors and the advancement of immunotherapy offer a fresh therapeutic approach for patients with Small Cell Lung Cancer. As the understanding of biomarkers in Small Cell Lung Cancer continues to expand within the field of oncology, the outcomes for patients with Small Cell Lung Cancer may eventually align more closely with those observed in the non-Small Cell Lung Cancer population.
Key Small Cell Lung Cancer companies such as Ipsen Biopharmaceuticals (Onivyde), Bristol-Myers Squibb (BMS-986012), Xcovery Holding Company (Vorolanib), EpicentRx (RRx-001), Amgen (AMG 757), and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Small Cell Lung Cancer.
Small Cell Lung Cancer Drugs Uptake
This section focuses on the uptake rate of potential Small Cell Lung Cancer drugs expected to be launched in the Small Cell Lung Cancer market during 2020–2034. For example, the bispecific T-cell engager tarlatamab (AMG 757) combining the binding specificities of DLL3 and CD3 is currently in a Phase II pivotal DeLLphi-301 study in Small Cell Lung Cancer. Based on the safety and efficacy data, existing and emerging competitors along with order of entry of this product, it is expected to undergo a specific type of uptake, which could vary from slow, medium and fast uptake in a specific number of years to attain its peak.
Further detailed analysis of emerging therapies drug uptake in the report…
Small Cell Lung Cancer Pipeline Development Activities
The Small Cell Lung Cancer therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes the Small Cell Lung Cancer companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Small Cell Lung Cancer drug market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Small Cell Lung Cancer emerging therapies.
KOL Views
To remain in line with the evolving Small Cell Lung Cancer market trends, we take Industry experts opinion working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on SCLC evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, include Medical/scientific writers, Medical Oncologists, Pulmonologists and Professors, Chief of Thoracic Service at the Memorial Sloan Kettering Cancer Center, and Others.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, Texas, UT Southwestern Medical Center in Dallas, Cancer Research UK Barts Centre in London, LUNGevity Foundation, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns, acceptability and accessibility issues along with Small Cell Lung Cancer market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging Small Cell Lung Cancer therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Small Cell Lung Cancer Market Access and Reimbursement
Lurbinectedin is cost-effective when compared with other second-line options for Small Cell Lung Cancer. Even though the acquisition cost of lurbinectedin is greater, this is offset by the lower cost of managing myelosuppression, when compared with other commonly used second-line therapies. Lurbinectedin is not only safe and effective but is also cost-effective in the care of patients with relapsed Small Cell Lung Cancer.
The Small Cell Lung Cancer market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
What’s new in 2023
- The results of a Phase I trial (NCT03639194) for ABBV-011, an antibody-drug conjugate (ADC) that targets SEZ6, were presented during the 2023 ASCO Annual Meeting. The trial demonstrated promising outcomes in patients with relapsed or refractorySmall Cell Lung Cancer.
- MAIA Biotechnology reported that in February 2023, the US FDA granted two orphan drug designations to THIO, an anti-cancer agent, based on its preclinical efficacy inSmall Cell Lung Cancer.
- Jubilant Therapeutics announced in January 2023 that JBI-802 received an orphan drug designation from the FDA for the treatment ofSmall Cell Lung Cancer in patients.
- Inspirna's press release in January 2023 highlighted that Abequolixron (RGX-104) exhibited early clinical activity when combined with docetaxel in heavily-pretreated patients withSmall Cell Lung Cancer, according to interim study results.
Scope of the Small Cell Lung Cancer Market Report
- The Small Cell Lung Cancer market report covers a segment of key events, an executive summary, descriptive overview ofSmall Cell Lung Cancer, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of theSmall Cell Lung Cancer market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Small Cell Lung Cancer treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Small Cell Lung Cancer market.
Small-cell Lung Cancer Market Report Insights
- Small Cell Lung Cancer Patient Population
- Small Cell Lung Cancer Therapeutic Approaches
- Small cell Lung Cancer Pipeline Analysis
- Small cell Lung Cancer Market Size
- Small Cell Lung Cancer Market Trends
- Existing and future Small Cell Lung Cancer Market Opportunity
Small-cell Lung Cancer Market Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Small-cell Lung Cancer Epidemiology Segmentation
- Key Cross Competition
- Small Cell Lung Cancer Conjoint analysis
- Small Cell Lung Cancer Drugs Uptake
- Key Small Cell Lung Cancer Market Forecast Assumptions
Small Cell Lung Cancer Market Report Assessment
- Current Small Cell Lung Cancer Treatment Practices
- Unmet Needs
- Small Cell Lung Cancer Pipeline Product Profiles
- Small Cell Lung Cancer Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
- What was the Small Cell Lung Cancer market size, the market size by therapies, and market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors for this growth?
- How will DLL3 Targeted T-Cell Engager as a new class affect the treatment paradigm inSmall Cell Lung Cancer?
- What kind of uptake will PD-L1 inhibitors witness in the coming 11 years?
- Why in the case of platinum-refractory (PR) patients, usage of platinum-based therapy can be seen especially in the case of Japan?
- What will be the impact of Lurbinectedin’s formulation patent expiry in 2028?
- Which class is going to be the largest contributor in 2032?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the Small Cell Lung Cancer market dynamics and subsequent analysis of the associated trends?
- What are the disease risk, burdens, and unmet needs of Small Cell Lung Cancer? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Small Cell Lung Cancer?
- What is the historical and forecasted Small Cell Lung Cancer patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What are the current options for the treatment of Small Cell Lung Cancer? What are the current treatment guidelines for the treatment of Small Cell Lung Cancer in the US and Europe?
- How many emerging SCLC therapies are in the mid-stage and late stage of development for the treatment of Small Cell Lung Cancer?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing SCLC therapies?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focusing on reimbursement policies.
Reasons to buy
- The SCLC Market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving theSmall Cell Lung Cancer Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Small Cell Lung Cancer market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Small Cell Lung Cancer companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet need of the existing Small Cell Lung Cancer therapeutics market so that the upcoming players can strengthen their development and launch strategy.